
A clinic-based tablet application to support safer conception among HIV serodiscordant couples in Kenya: feasibility and acceptability study
Introduction
HIV transmission within stable, HIV serodiscordant partnerships accounts for a substantial fraction of new HIV infections in sub-Saharan Africa (1). Serodiscordant couples face risk of sexual and perinatal HIV transmission when attempting to conceive through vaginal sex, yet it is not uncommon for couples to knowingly risk HIV transmission to meet their reproductive goals (2-6). In qualitative work with HIV serodiscordant couples in East Africa, couples understood that “getting a child could be risky” but they still intended to conceive because of personal desires for children, external pressure from others, and fears about experiencing stigma or relationship dissolution if they failed to conceive (3).
Safer conception strategies, including antiretroviral therapy (ART) use by the HIV-infected partner, pre-exposure prophylaxis (PrEP) by the HIV-uninfected partner, condomless sex restricted to days with peak fertility, testing and treatment for genital tract infections, voluntary medical male circumcision, vaginal self-insemination, and assisted reproductive interventions (e.g., sperm washing) can minimize HIV risk for serodiscordant couples while supporting pregnancy desires (7-11). These options vary in cost and acceptability, and timed condomless sex, ART, and PrEP are the most commonly reported strategies used by serodiscordant couples in Africa (8,12,13). However, couples often describe difficulty determining ovulation timing, tracking fertility indicators (e.g., basal body temperature, cervical mucus characteristics), and sustaining timed condomless sex, ART, and PrEP use while attempting pregnancy (12,14,15). Moreover, research has shown that couples face stigma and healthcare provider bias when seeking safer conception counseling and providers may need additional tools to foster a supportive atmosphere while accurately communicating with a couple about their individual HIV transmission risk (13,14,16,17).
Mobile health (“mHealth”) strategies, including short message service (SMS) and tablet-based applications (“apps”), can be integrated with safer conception interventions to predict upcoming peak fertility days and enhance provider-couple communication. In Kenya, several studies have demonstrated the acceptability, feasibility, and effectiveness of SMS messages aimed at increasing rates of HIV testing (18) and ART adherence (19-21), improving sexual behavior measurement (22,23), and increasing healthcare worker adherence to HIV treatment guidelines (24). mHealth interventions have also been used to deliver information regarding menstrual cycle timing and family planning methods to address health systems challenges related to service availability and efficiency during clinic visits (25-29). However, these mHealth programs are primarily directed at patients rather than healthcare providers, and none have been developed to specifically support safer conception among HIV serodiscordant couples (30). They also primarily rely on SMS for information delivery, and few mHealth interventions in sub-Saharan Africa include mobile application components which may be more effective at communicating health information with patients, visualizing data, and improving reproductive health outcomes than SMS alone (30).
To address this gap, we designed a novel mHealth tablet application for providers to use during clinic-based safer conception counseling sessions among HIV serodiscordant couples in Kenya. The application draws on individual data from daily fertility assessment via SMS messages and PrEP and ART adherence information gathered during clinic visits to prepare HIV serodiscordant couples for timed condomless sex, enhance clinician-couple communication, and minimize HIV transmission risk while couples are trying to become pregnant. Here, we report mixed methods data on the feasibility and acceptability of the mHealth intervention.
Methods
Setting, participants, and procedures
The Safer Conception Intervention for Partners (SCIP, clinicaltrials.gov #NCT03030768) pilot study was conducted from March 2016 through April 2018 in a clinical research site in Thika, Kenya (31). The main objective was to develop and pilot test a comprehensive safer conception intervention for HIV serodiscordant couples which included: (I) SMS fertility tracking and ovulation prediction, (II) daily oral PrEP [co-formulated emtricitabine/tenofovir disoproxil fumarate (FTC/TDF)] for HIV-negative partners, (III) in-depth counseling to accompany publicly-provided ART for HIV-positive partners, (IV) counseling on PrEP, ART adherence, and timing condomless sex to peak fertility periods, and (V) diagnostic testing and treatment for sexually transmitted infections. We also provided counseling on additional safer conception options (e.g., self-insemination) and off-site referrals for male circumcision and fertility care.
HIV serodiscordant couples were recruited through partnership with HIV testing and counseling (HTC) centers and ART clinics near Thika, Kenya, distribution of educational materials to couples on the benefits of couples-based HTC, and talking with support groups for HIV-serodiscordant couples. Eligible couples were ≥18 years old (women were between 18 and 49 years old), not pregnant at enrollment, sexually active, literate in English, Kiswahili, or Kikuyu, able to send and receive SMS messages, planned to remain a couple for at least one year, and had immediate desires to become pregnant. Both members of the couple were required to have their own mobile phone for personal use (a smartphone was not required) that operated using a telecom network supported by the study SMS platform. PrEP was offered to HIV-uninfected participants at enrollment and throughout follow-up. All participants attended monthly study visits until pregnancy and quarterly visits thereafter and were followed for one year or until the end of pregnancy. At each visit, they completed standardized, interviewer-administered questionnaires (in their preferred language) to capture data on sociodemographic factors, sexual behavior, fertility intentions, and contraceptive use. Women were also given Clearblue ovulation prediction kits (OPKs; Swiss Precision Diagnostics GmbH) and digital thermometers to measure basal body temperature throughout follow-up (32,33). They were instructed on the use of OPKs and thermometers during the enrollment visit and completed role plays with trained study counselors at enrollment and one-month study visits to demonstrate their ability to use these devices. Re-trainings were conducted at follow-up visits as needed. Prior to pregnancy, counseling sessions included the use of a clinic-based tablet application (“SCIP-App”). SCIP-App was accessed by providers during sessions and included participant data from the period between visits; participants did not have access to SCIP-App on their mobile devices outside of the clinic.
SCIP-App development
SCIP-App was developed through a five-stage, user-centered design process: (I) iterative problem scoping and stakeholder engagement; (II) healthcare provider workflow mapping for clinic visits; (III) reviewing current knowledge and practice about peak fertility timing among HIV serodiscordant couples in Kenya; (IV) rapid application prototyping; and (V) reviewing the prototype with HIV providers, female members of HIV serodiscordant couples, data managers, and investigators at the study clinic followed by final adjustments (34,35). SCIP-App code is freely available (https://github.com/SCIP-app/androidApp) and was designed for use with experienced counselors and clinicians.
SCIP-App incorporates data from 3 sources: female participants’ fertility information captured in daily SMS messages; HIV viral load data from HIV-infected partners with samples drawn during quarterly clinic visits until viral suppression and every six months thereafter; and PrEP adherence information generated from medication event monitoring system (MEMS) caps on pill bottles provided to HIV-uninfected partners and downloaded during monthly clinic visits following PrEP initiation (Figure 1). Six daily SMS messages asked questions about menses (“Are you on your monthly periods today?”), basal body temperature (“What was your basal body temperature this morning?”), OPK results (“positive” or “negative”), vaginal mucus (“Is your vaginal mucus sticky, like nasal mucus, today?”), sexual activity (“Since this time yesterday, did you have sex?”), and condom use (“Did you use a condom for all sex acts?”). Participants also received a message at the end of each survey reminding them that they could delete the messages to protect their privacy. SMS surveys were used to predict upcoming peak fertility days and guide counseling recommendations for timed condomless sex in the next month. We defined “peak fertility days” as the four days prior to the date of next ovulation, estimated by: (I) calculating average menstrual cycle length using participant’s self-reported last menstrual period dates from the last 3 months; (II) estimating the approximate ovulation day for each cycle using SMS data on OPK results; (III) calculating the average luteal phase length as the difference between the day of ovulation and the onset of next menses; and (IV) using these data to predict subsequent onsets of menses and next ovulation days.
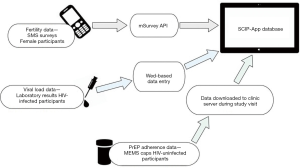
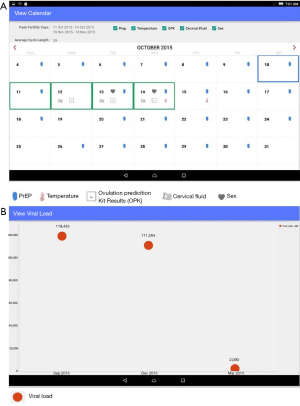
Files with viral load and MEMS data were saved to the tablet’s memory card and manually imported into the SCIP-App database. SCIP-App automatically pulled the SMS responses from the survey application programming interface (API) into the application’s database. These data were displayed in SCIP-App via an interactive safer conception calendar which included upcoming peak fertility days and symbols on each day to represent fertility indicator data, PrEP use, and condomless sex in the prior month (Figure 2A). A separate screen depicted viral load results of the HIV-infected partner graphically (Figure 2B). Calendar and viral load data were linked to specific couple numbers and were reviewed when both members of the couple attended the clinic together. However, when only one member of the couple attended a visit, SCIP-App screen(s) with data from the absent member of the couple were hidden to maintain confidentiality. Other requests from a member of the couple to keep data confidential from their study partner were also honored, although this occurred infrequently.
Data management and analysis
Daily SMS messages were sent and managed by mSurvey, a Nairobi-based mobile data collection company (https://msurvey.co/). Female participants received the six daily SMS messages from the day after enrollment until pregnancy, and they were able to choose a preferred time of day to receive these surveys. Participants who miscarried were given the option to restart SMS messages when they were ready to attempt pregnancy again. SMS data were downloaded weekly by study data managers to track SMS outages and response rates. Participants who repeatedly missed surveys were contacted by the study team to understand barriers to survey completion and troubleshoot problems.
We incorporated a Google Analytics platform into SCIP-App to collect data on activity, including application openings and crashes. These data were tracked by clinician login credentials rather than study participant identification numbers.
We used descriptive statistics to summarize the study sample, SMS delivery and response rates, and SCIP-App usage during follow-up. These analyses were conducted using SAS 9.4 (Cary, North Carolina, USA) and Google Analytics (Mountain View, California, USA, http://google.com/analytics).
Qualitative data collection and analysis
We conducted in-depth interviews with a purposive sample of both members of 19 couples and 5 clinical research staff to explore the acceptability and feasibility of using mHealth tools to promote safer conception. Semi-structured interview guides, informed by prior research and literature reviews in the fields of HIV, reproductive health, and mHealth research, were developed collaboratively by team members. Interviewers piloted the guides with study staff and final guides were translated into Kiswahili and Kikuyu. The guides included probes related to SMS message delivery (e.g., message timing, difficulties receiving or sending messages) and participants’ opinions about the use of SCIP-App to track fertility, viral load, and PrEP use data. Key informant interview guides also included questions about the provider’s experience using SCIP-App during counseling and attitudes about it as a tool to engage with participants.
Interviews were conducted by two experienced Kenyan social scientists (one male and one female, gender-matched to participants when possible). Participants were recruited during routine study visits and were informed that the interview would not affect their SCIP study participation or clinical care. Interviews were conducted in English, Kiswahili, and/or Kikuyu, based on participant preferences, and lasted approximately 45 minutes. Data collection took place in a private area of the clinic. All interviews were audio-recorded, transcribed, and translated into English.
Qualitative data were analyzed using a modified constant comparative approach (36). We developed an initial codebook based on the interview guide and transcripts, and the codebook was iteratively refined through coding and group discussions. Transcripts were imported into Dedoose software (version 7.0.23, Los Angeles, CA, USA: Sociocultural Research Consultants, LLC) for analysis. Approximately 20% of transcripts were coded by two members of the study team (JV, NT), and coding disagreements were resolved through discussion with the team until consensus was reached. The remaining transcripts were coded by one member of the study team (JV or NT). Five team members (JV, NT, MK, KN, RH) identified key themes from the data by reading transcripts, comparing and contrasting themes by participants’ gender and HIV status, and using memo writing and diagramming techniques to identify similarities and differences across interviews (37). We also selected representative quotations pertaining to each main theme.
Ethical approval
Protocols were approved by ethical review boards at the University of Washington and Kenya Medical Research Institute (KEMRI). All participants provided written informed consent in their preferred languages.
Results
Participant characteristics
We enrolled 74 HIV serodiscordant couples, including 34 (45.9%) couples with an HIV-infected female partner. The median age of the female partners at enrollment was 30 years [interquartile range (IQR), 27–35 years; Table 1). All HIV-uninfected participants accepted PrEP at enrollment and all participants living with HIV were on ART by their 1-month follow-up visit. A total of 143 (96.6%) participants had at least one follow-up visit, and 47 pregnancies and 0 HIV infections were diagnosed during follow-up.

Full table
SMS survey completion and SCIP-App engagement
There were 17,360 daily surveys planned for delivery from March 24, 2016 to April 30, 2018. Of these, 16,905 SMS surveys (97.4%) were sent and each participant received a median of 259 surveys (IQR, 110–332) during the 2-year study period. A small number of surveys were not sent as expected due disruptions in service delivery from the mSurvey SMS platform (N=248; 1.4% of 17,360 planned surveys) or the Safaricom service provider (N=184; 1.1%), or because participants were mistakenly deactivated from surveys (N=23; 0.1%).
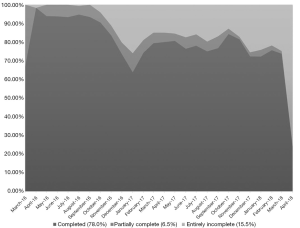
Participants completed 13,181 surveys (78.0% of those sent) with a median of 167 fully completed surveys (IQR, 74–299) per participant. Approximately 6.5% of sent surveys were partially completed (participants missed a response to 1–5 items) and 15.5% were entirely incomplete (missed responses to all items). The most commonly missed item was basal body temperature, with 3,548 surveys missing a temperature value (21.0% of all surveys sent). We observed decreased SMS completion during the annual end-of-year holiday period (Figure 3). More than three-quarters of participants completed at least 75% of their SMS messages (N=58; 77.3%) and more than half completed at least 90% of their messages (N=40; 53.3%). SMS completion rates were slightly higher among participants who became pregnant during follow-up [mean of 80.2% of surveys completed; standard deviation (SD): 23.8] than those who did not become pregnant (mean of 76.2% of surveys completed; SD: 27.5).
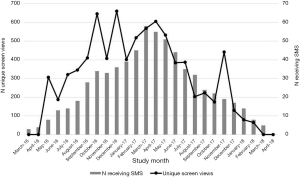
SCIP-App was opened by counselors 1,806 times from March 2016 to March 2018 during 1,179 SCIP study visits. The number of openings exceeded the number of visits because counselors opened SCIP-App to review participants’ data, check that data had been uploaded correctly, and periodically test SCIP-App performance. Application use generally corresponded with the number of participants enrolled and receiving SMS messages during a given study month, particularly after the counselors had opportunities to practice using the application during the first few months of the study (Figure 4). The calendar view page was opened 1,691 times (93.6% of 1,806 application sessions) and the viral load page was opened 377 times (20.9%).
Qualitative findings
We conducted interviews with 19 couples (including 10 couples with an HIV-uninfected female partner), 3 nurse counselors, and 2 clinicians. Qualitative data revealed that the SMS messages and SCIP-App were feasible and acceptable for fertility tracking and were recommended for broader use within their community. Four main themes emerged as described below (Table 2).
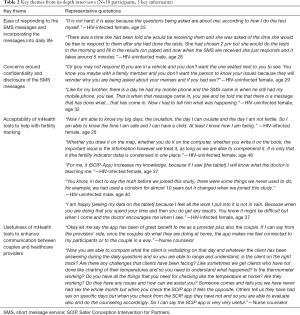
Full table
Ease of responding to SMS and incorporating SMS into daily life
Participants overwhelmingly found the messages easy to respond to because they were asking about “how I do the tests”. Most participants reported that they would typically conduct the tests in the morning and record their answers on paper, as they had done for fertility tracking prior to joining the study, and then they would be able to quickly respond to the SMS messages in the evening when they had privacy: “(Responding takes) maybe two minutes. But it depends on when you answer it because sometimes you have to go back to the book where you have signed in morning. You have to go to look for that notes so that you can send the appropriate answer. If I was in the bus or I was driving I won’t get home until in an hours’ time or maybe I am busy with other people…but I will remember to answer it before midnight most of the time.”—HIV-uninfected female, age 48.
The basal body temperature question was the most difficult to respond to because it required participants to type in multiple digits and include a decimal place. This finding contextualizes quantitative data on the frequency of missing basal body temperature data: “The process of sending SMS, I am asked if I am on my menses. If I am on my menses I respond with the appropriate number; either number 1, number 2 or number 3. The second one is my basal body temperature that I take every morning. I send the one I took in the morning at night…The one for basal temperature (is the most challenging) because one has to key in the number which takes time and sometimes you are sleepy.”—HIV-uninfected female, age 42.
They appreciated being able to choose their preferred language (“I chose Kikuyu so that I can understand it better”, HIV-infected female, age 37). Participants did report technical issues receiving SMS messages if “Safaricom is having problems” or they were “in rural areas where there are problems with electricity”. Messages could be reportedly delayed anywhere between a few hours and three days, but “when there are delays someone is informed”. Participants appreciated communication with the study staff about Safaricom service outages.
Concerns around confidentiality and disclosure of SMS messages
A few participants reported concerns about answering the messages in front of other people because they did not want questions about sexual behavior to be seen. However, these concerns were relatively minimal and were not specifically related to HIV status disclosure. Concerns around confidentiality also arose when participants visited the clinic and saw their information on the tablet application, although this appeared to be more of an issue among older participants and those who did not have smart phones: “We have the ones (participants) who don’t have smart phones so the whole issue of the app is a new thing and then the ones who worry about their privacy, yes because part of the question is did you have sex today then now it will be taken there and shown on the app so people ask about their privacy.”—Clinician, key informant at the study clinic.
One participant described an incident where her brother saw the messages on her phone and then she had to tell him about the study, but this was the only instance of unintended disclosure reported. Several participants said they deleted the SMS messages from their phones or requested to receive the messages in the evening when they were likely to be alone in order to avoid unintended disclosure. One instance of verbal and physical abuse related to the SMS messages was reported during the study, when a male partner mistook a message as being from a former partner.
Acceptability of mHealth tools to help with fertility tracking
Participants found the SMS and SCIP-App interventions to be helpful at telling them their “big days, the days I can ovulate” and providing them with improved knowledge and guidance on when they could have sex without a condom which they had not felt comfortable doing prior to the study. Women reported that the SMS messages “reminded me to do the [ovulation] tests” and served as a useful record of their fertility indicators over time, which they appreciated if they did not use paper records or if they forgot to update their paper records: “It is very beneficial because sometimes even if I did not record in my calendar I refer from the SMS. Like let’s say if yesterday I did not remember to record, today I check what I sent from the SMS because for something like temperature sometimes someone forgets the reading.I keep the SMS for like 2 days so that even if I forget to record on the calendar I will refer from the SMS.”—HIV-uninfected female, age 39.
In addition, the daily messages made participants feel like “there are people who are concerned about me” and increased their satisfaction with clinic services and engagement in the safer conception intervention: “When I send them (the SMS) I feel like I am with the doctor and that is a very good connection.”—HIV-uninfected female, age 42.
SCIP-App was also useful for retrospectively tracking participants’ fertility indicators, PrEP use, and viral load, predicting upcoming peak fertility days, and improving their understanding of their fertility during monthly clinic visits. One HIV-uninfected woman who already used a paper calendar to record her fertility data in between clinic visits said that the application “reaffirms what you already have an idea about” and she appreciated the application because “it had all the information condensed in one place”. Other participants enjoyed looking at the application with the counselor every month as a way to “increase my knowledge” about fertility indicators and share their data back with them [“I am happy (seeing my data on the tablet) because I feel all the work I put into it is not in vain”, HIV-infected female, age 37]. Men found SCIP-App to be helpful for “understanding a woman’s fertility” and they reported that the mHealth tools “educated” them and helped them to communicate with their partner about peak fertility timing and safer conception strategies.
Usefulness of mHealth tools to enhance communication between couples and providers
All key informants reported that the SMS and SCIP-App enhanced their counseling experiences because it improved feelings of connection with participants. Specifically, just as participants valued receiving SMS messages every day as a proxy for healthcare staff reaching out to them, providers enjoyed seeing “what they (participants) are doing at home” and receiving granular data on participants’ fertility indicators and sexual behavior. They used the SMS data to assist with counseling. For example, if a participant did not respond to SMS questions for several days or consistently missed the question about basal body temperature, study staff would probe about missing data and assess participants’ challenges with the messages. In a non-judgmental way, they could ask participants about discrepancies in their data, such as days when they reported having condomless sex via SMS data but did not report that during the clinic visit. Staff were able to show data to both members of the couple and appreciated the opportunity to transparently display peak fertility timing information for participants based on their SMS responses: “I love that I can show the partner (HIV-uninfected participant) and the index (HIV-infected participant)…when we say the very fertile [days], this is what we look at the app. On that app you see we have high temperature, the OPK kit is positive so we actually use that information from you to help you.”—Clinician, key informant.
Finally, they used the SCIP-App data in monthly PrEP and ART adherence counseling sessions, which was particularly helpful in the context of this safer conception intervention, and they reported regular use of the viral load view as well as the calendar view: “You cannot do adherence counseling before (getting) information about how she has been taking PrEP. So the app is of importance; it aids us to probe more and know more. Perhaps from the app maybe we are seeing some 5 days when the drug was not taken. Finally they open up and tell you actually there is a day I was not at home and that is when I missed those two days. So actually they end up remembering about some days and telling us more about them.”—Nurse counselor, key informant.
Discussion
In this pilot safer conception study with HIV serodiscordant couples in Kenya, a combined SMS and fertility tracking tool enhanced participant and provider implementation of a multi-faceted set of HIV prevention strategies. Daily SMS messages were an acceptable and feasible means to track fertility indicators and we observed high response rates and minimal SMS service delivery issues accompanied by qualitative data illustrating that participants found it easy to respond to the messages, had minimal concerns with confidentiality, and felt supported in achieving fertility goals. The SMS data were successfully integrated into a tablet-based application, which was regularly used during counseling sessions and included information on fertility indicators, PrEP use, and HIV viral load. Participants reported that the application improved their understanding of fertility and HIV medication data and increased their motivation to track their fertility indicators. Key informants reported that the application enhanced patient-provider communication.
Prior studies have similarly demonstrated the acceptability and feasibility of using daily or weekly SMS messages to collect sexual behavior data among women in sub-Saharan Africa (18,38,39). For example, one recent study conducted in South Africa had an 82% response rate to daily SMS messages and found that women reported higher frequency of vaginal sex via SMS than paper surveys, suggesting that daily SMS messages can reduce biases in sexual behavior reporting (38). No published study or program has examined the use of daily SMS messages for collecting fertility indicator data in sub-Saharan Africa and our high survey response rates provide encouraging evidence about the acceptability of using SMS to collect fertility data in conjunction with sexual behavior data.
In addition to considerations of SMS content, SMS message frequency is a key component of mHealth intervention acceptability and feasibility. One SMS-based intervention for ART adherence found that weekly SMS messages may be more effective than daily messages, in part because the weekly messages may minimize SMS intrusiveness and response fatigue (20,40). We did not observe a notable decrease in response rates over time and participants reportedly enjoyed receiving daily SMS messages as a reminder to track their fertility indicators. Daily SMS messages, rather than weekly messages, may have been appropriate in this cohort because they made participants feel like a provider was reaching out to them on a regular basis and cared about their fertility, which has been previously associated with SMS acceptability in studies among HIV-infected adults in Uganda (41,42). Couples who are trying to achieve pregnancy may be highly motivated and weekly SMS reminders would not be sufficient for appropriately tracking a fertile window. In addition, our participants reported minimal confidentiality and disclosure concerns, which has also been linked with SMS intervention success (43-46).
The majority of mHealth studies in sub-Saharan Africa have used SMS messages for health education, reminder messaging, and data collection; however, these strategies may have minimal effectiveness if they are not well integrated into existing healthcare systems or paired with other health provider and systems-based technologies such as provider-facing mHealth tools or patient-facing smart phone applications (25,30). Although patient-facing phone and tablet applications for fertility tracking exist worldwide, studies have shown low uptake of these tools in settings with low knowledge about fertility indicators and where women report skepticism of the applications’ ability to correctly predict ovulation cycles (47). Women in our cohort appreciated being able to review their application data with a provider and were reassured by seeing their SMS data in the application dashboard, which likely also contributed to their steady SMS response rates over study follow-up. Clinic-based tablet applications may be especially important for fertility tracking among HIV serodiscordant couples who desire provider reassurance and counseling about their peak fertility timing for condomless sex (14). In addition, HIV providers frequently report desires for increased training on safer conception counseling and have described difficulties integrating HIV prevention messages into family planning and reproductive health service delivery (48-50). SCIP study clinicians and counselors regularly used the clinic-based tablet application during counseling sessions and found that it enhanced patient-provider communication. Similar results have been shown in Tanzania, where a clinic-based tablet application was successfully used to capture HIV testing and pregnancy-related health information and was readily adopted by providers (51).
The strengths of this study include the user-centered design process for SCIP-App development and the use of both quantitative and qualitative data to triangulate our findings. SMS response rates were high and we closely monitored SMS delivery to detect outages, diagnose delivery problems, and inform participants about technical problems. Our approach also linked patient-based SMS data collection with a provider-facing mHealth tool. Limitations of this study included our small sample size and the lack of a comparison group which limited our ability to make conclusions about the utility of SMS messages or SCIP-App compared with standard-of-care counseling. We did not collect data on method of couple recruitment and couples who were recruited from ART clinics may have had a different level of SMS engagement than those who were recruited from HTC centers and were newly HIV diagnosed or those who presented to the clinic after reading educational outreach materials. We were not able to disaggregate data on SCIP-App usage by participant or restrict the dataset only to instances when the application was opened during a counseling session. Interviews were conducted with a purposive sample of study participants who may not have been representative of the larger SCIP sample with regard to engagement in the SMS messages, SCIP-App, and overall study procedures. However, we mitigated this issue by conducting interviews until we reached saturation of key themes. Finally, our findings may not be generalizable to populations with lower literacy levels or less familiarity with SMS and tablet applications.
Conclusions
In conclusion, mHealth tools including daily SMS surveys and clinic-based tablet applications are feasible and acceptable for promoting safer conception among HIV serodiscordant couples in Kenya. These findings highlight the potential for innovative technology-based programs to meet the fertility needs of HIV serodiscordant couples and better equip healthcare providers with resources to enhance counseling and communication with patients. Additional research is necessary to examine the effectiveness of these mHealth approaches for increasing fertility rates among HIV serodiscordant couples, explore key mediators in the pathway between program delivery and health outcomes, and scale-up successful mHealth services outside of research settings. The SCIP study provides an exciting example of strategies for integrating SMS data collection with provider-facing tablet application to improve patient engagement and enhance HIV prevention and reproductive health in East Africa.
Acknowledgements
The authors thank the couples who participated in the study. We would also like to acknowledge the teams at the study site and the University of Washington that supported data collection and management for this work, and the team at mSurvey who supported SMS data collection, including: Harald Haugen, Susan Morrison, Jennifer Morton, Katherine K. Thomas, Peter Mogere, Peter Michira, Jacinta Nyokabi, Edith Kimani, Richard Momanyi, John Njoroge, Peterson Mwaniki, Lawrence Mwihaki, Grace Kimemia, Linda Oluoch, Bhavna Chohan, Edwin Mugo, James Munyao, Snaida Ayub, Lawrence Mwaniki, Sarah Mbugua, Njambi Njuguna, Elizabeth Irungu.
Funding: This work was supported by the Eunice Kennedy Shriver National Institute for Child Health and Human Development of the US National Institutes of Health (grant R00 HD076679) and the Fogarty International Center (grant R21 TW009908). J Velloza was supported by the National Institute of Mental Health of the US National Institutes of Health (grant F31 MH113420).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: Gilead Sciences donated the PrEP medication but had no role in data collection or analysis. The results and interpretation presented here do not necessarily reflect the views of the study funders.
References
- Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet 2008;371:2183-91. [Crossref] [PubMed]
- Matthews LT, Crankshaw T, Giddy J, et al. Reproductive decision-making and periconception practices among HIV-positive men and women attending HIV services in Durban, South Africa. AIDS Behav 2013;17:461-70. [Crossref] [PubMed]
- Ngure K, Baeten JM, Mugo N, et al. My intention was a child but I was very afraid: Fertility intentions and HIV risk perceptions among HIV serodiscordant couples experiencing pregnancy in Kenya. AIDS Care 2014;26:1283-7. [Crossref] [PubMed]
- Kisakye P, Akena WO, Kaye DK. Pregnancy decisions among HIV-positive pregnant women in Mulago Hospital, Uganda. Cult Health Sex 2010;12:445-54. [Crossref] [PubMed]
- Brubaker SG, Bukusi EA, Odoyo J, et al. Pregnancy and HIV transmission among HIV-discordant couples in a clinical trial in Kisumu, Kenya. HIV Med 2011;12:316-21. [Crossref] [PubMed]
- Awiti Ujiji O, Ekström AM, Ilako F, et al. “I will not let my HIV status stand in the way.” Decisions on motherhood among women on ART in a slum in Kenya- a qualitative study. BMC Womens Health 2010;10:13. [Crossref] [PubMed]
- Matthews LT, Mukherjee JS. Strategies for harm reduction among HIV-affected couples who want to conceive. AIDS Behav 2009;13:5-11. [Crossref] [PubMed]
- Vernazza PL, Graf I, Sonnenberg-Schwan U, et al. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS 2011;25:2005-8. [Crossref] [PubMed]
- Zafer M, Horvath H, Mmeje O, et al. Effectiveness of semen washing to prevent human immunodeficiency virus (HIV) transmission and assist pregnancy in HIV-discordant couples: a systematic review and meta-analysis. Fertil Steril 2016;105:645-655.e2. [Crossref] [PubMed]
- Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med 2011;365:493-505. [Crossref] [PubMed]
- Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 2007;369:643-56. [Crossref] [PubMed]
- Wagner GJ, Linnemayr S, Goggin K, et al. Prevalence and correlates of use of safer conception methods in a prospective cohort of Ugandan HIV-affected couples with fertility intentions. AIDS Behav 2017;21:2479-87. [Crossref] [PubMed]
- Schwartz SR, Bassett J, Holmes CB, et al. Client uptake of safer conception strategies: implementation outcomes from the Sakh’umndeni Safer Conception Clinic in South Africa. J Int AIDS Soc 2017;20:21291. [Crossref] [PubMed]
- Ngure K, Kimemia G, Dew K, et al. Delivering safer conception services to HIV serodiscordant couples in Kenya: perspectives from healthcare providers and HIV serodiscordant couples. J Int AIDS Soc 2017;20:21309. [Crossref] [PubMed]
- Friedman E, Orlando MS, Anderson J, et al. “Everything I needed from her was everything she gave back to me:” An evaluation of preconception counseling for US HIV-serodiscordant couples desiring pregnancy. Womens Health Issues 2016;26:351-6. [Crossref] [PubMed]
- Mindry D, Wanyenze RK, Beyeza-Kashesya J, et al. Safer conception for couples affected by HIV: structural and cultural considerations in the delivery of safer conception care in Uganda. AIDS Behav 2017;21:2488-96. [Crossref] [PubMed]
- Ngure K, Heffron R, Curran K, et al. I Knew I Would Be Safer. Experiences of Kenyan HIV Serodiscordant Couples Soon After Pre-Exposure Prophylaxis (PrEP) Initiation. AIDS Patient Care STDS 2016;30:78-83. [Crossref] [PubMed]
- Njuguna N, Ngure K, Mugo N, et al. The effect of Human Immunodeficiency Virus Prevention and reproductive health text messages on Human Immunodeficiency Virus testing among young women in rural Kenya: A pilot study. Sex Transm Dis 2016;43:353-9. [Crossref] [PubMed]
- Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 2010;376:1838-45. [Crossref] [PubMed]
- Pop-Eleches C, Thirumurthy H, Habyarimana JP, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS 2011;25:825-34. [Crossref] [PubMed]
- Thakkar J, Kurup R, Laba TL, et al. Mobile telephone text messaging for medication adherence in chronic disease: A meta-analysis. JAMA Intern Med 2016;176:340-9. [Crossref] [PubMed]
- Curran K, Mugo NR, Kurth A, et al. Daily short message service surveys to measure sexual behavior and pre-exposure prophylaxis use among Kenyan men and women. AIDS Behav 2013;17:2977-85. [Crossref] [PubMed]
- Haberer JE, Ngure K, Muwonge T, et al. Brief report: Context matters: PrEP adherence is associated with sexual behavior among HIV serodiscordant couples in East Africa. J Acquir Immune Defic Syndr 2017;76:488-92. [Crossref] [PubMed]
- Zurovac D, Sudoi RK, Akhwale WS, et al. The effect of mobile phone text-message reminders on Kenyan health workers’ adherence to malaria treatment guidelines: a cluster randomised trial. Lancet 2011;378:795-803. [Crossref] [PubMed]
- Ashcroft N, Shelus V, Garg H, et al. Implementation of CycleTel family advice: an SMS-based service to provide family planning and fertility awareness information in India. mHealth 2017;3:20. [Crossref] [PubMed]
- van Dijk MR, Oostingh EC, Koster MPH, et al. The use of the mHealth program Smarter Pregnancy in preconception care: rationale, study design and data collection of a randomized controlled trial. BMC Pregnancy Childbirth 2017;17:46. [Crossref] [PubMed]
- L’Engle KL, Vahdat HL, Ndakidemi E, et al. Evaluating feasibility, reach and potential impact of a text message family planning information service in Tanzania. Contraception 2013;87:251-6. [Crossref] [PubMed]
- World Health Organization. Classificaton of digital health interventions v1.0: A shared language to describe the uses of digital technology for health. 2018 [cited 2018 Jul 11]. Available online: http://apps.who.int/iris/bitstream/handle/10665/260480/WHO-RHR-18.06-eng.pdf;jsessionid=1138C299E7F499BD2737F7F33A500BEC?sequence=1
- Unger JA, Ronen K, Perrier T, et al. Short message service communication improves exclusive breastfeeding and early postpartum contraception in a low- to middle-income country setting: a randomised trial. BJOG 2018;125:1620-9. [Crossref] [PubMed]
- Chen H, Chai Y, Dong L, et al. Effectiveness and appropriateness of mHealth interventions for maternal and child health: Systematic review. JMIR MHealth UHealth 2018;6:e7. [Crossref] [PubMed]
- Heffron R, Ngure K, Quame-Amaglo J, et al. Successful use of safer conception strategies resulting in high pregnancy rates and no HIV transmissions among Kenyan HIV discordant couples. Oral Presentation Session SY07.01 presented at: HIV Research for Prevention (HIVR4P) 2018;2018 Oct 21; Madrid, Spain.
- Guida M, Tommaselli GA, Palomba S, et al. Efficacy of methods for determining ovulation in a natural family planning program. Fertil Steril 1999;72:900-4. [Crossref] [PubMed]
- Behre HM, Kuhlage J, Gassner C, et al. Prediction of ovulation by urinary hormone measurements with the home use ClearPlan Fertility Monitor: comparison with transvaginal ultrasound scans and serum hormone measurements. Hum Reprod 2000;15:2478-82. [Crossref] [PubMed]
- McCurdie T, Taneva S, Casselman M, et al. mHealth consumer apps: the case for user-centered design. Biomed Instrum Technol 2012.Suppl:49-56. [Crossref] [PubMed]
- De Vito Dabbs A, Myers BA, Mc Curry KR, et al. User-centered design and interactive health technologies for patients. Comput Inform Nurs 2009;27:175-83. [Crossref] [PubMed]
- Glaser B, Strauss A. The Discovery of Grounded Theory, Strategies for Qualitative Research. Chicago, IL: Aldine Publishing Company, 1967.
- Miles M, Huberman A, Saldana J. Qualitative Data Analysis: A Methods Sourcebook. London, UK: SAGE Publications, 2013.
- Dietrich JJ, Lazarus E, Andrasik M, et al. Mobile phone questionnaires for sexual risk data collection among young women in Soweto, South Africa. AIDS Behav 2018;22:2312-21. [Crossref] [PubMed]
- Lim MSC, Sacks-Davis R, Aitken CK, et al. Randomised controlled trial of paper, online and SMS diaries for collecting sexual behaviour information from young people. J Epidemiol Community Health 2010;64:885-9. [Crossref] [PubMed]
- Baseman JG, Revere D, Painter I, et al. Public health communications and alert fatigue. BMC Health Serv Res 2013;13:295. [Crossref] [PubMed]
- Ware NC, Pisarski EE, Tam M, et al. The meanings in the messages: how SMS reminders and real-time adherence monitoring improve antiretroviral therapy adherence in rural Uganda. AIDS 2016;30:1287-94. [Crossref] [PubMed]
- Musiimenta A, Atukunda EC, Tumuhimbise W, et al. Acceptability and feasibility of real-time antiretroviral therapy adherence interventions in rural Uganda: Mixed-method pilot randomized controlled trial. JMIR MHealth UHealth 2018;6:e122. [Crossref] [PubMed]
- Rana Y, Haberer J, Huang H, et al. Short message service (SMS)-based intervention to improve treatment adherence among HIV-positive youth in Uganda: focus group findings. PLoS One 2015;10:e0125187. [Crossref] [PubMed]
- Baranoski AS, Meuser E, Hardy H, et al. Patient and provider perspectives on cellular phone-based technology to improve HIV treatment adherence. AIDS Care 2014;26:26-32. [Crossref] [PubMed]
- Siedner MJ, Santorino D, Haberer JE, et al. Know your audience: predictors of success for a patient-centered texting app to augment linkage to HIV care in rural Uganda. J Med Internet Res 2015;17:e78. [Crossref] [PubMed]
- Brown W, Giguere R, Sheinfil A, et al. Challenges and solutions implementing an SMS text message-based survey CASI and adherence reminders in an international biomedical HIV PrEP study (MTN 017). J Biomed Inform 2018;80:78-86. [Crossref] [PubMed]
- Starling MS, Kandel Z, Haile L, et al. User profile and preferences in fertility apps for preventing pregnancy: an exploratory pilot study. mHealth 2018;4:21. [Crossref] [PubMed]
- Mmeje O, Njoroge B, Akama E, et al. Perspectives of healthcare providers and HIV-affected individuals and couples during the development of a Safer Conception Counseling Toolkit in Kenya: stigma, fears, and recommendations for the delivery of services. AIDS Care 2016;28:750-7. [Crossref] [PubMed]
- Matthews LT, Bajunirwe F, Kastner J, et al. “I always worry about what might happen ahead”: Implementing safer conception services in the current environment of reproductive counseling for HIV-affected men and women in Uganda. BioMed Res Int 2016;2016:4195762. [Crossref] [PubMed]
- Goggin K, Hurley EA, Wagner GJ, et al. Changes in providers’ self-efficacy and intentions to provide safer conception counseling over 24 months. AIDS Behav 2018;22:2895-905. [Crossref] [PubMed]
- Bull S, Thomas DS, Nyanza EC, et al. Tanzania Health Information Technology (T-HIT) System: Pilot test of a tablet-based system to improve prevention of mother-to-child transmission of HIV. JMIR MHealth UHealth 2018;6:e16. [Crossref] [PubMed]
Cite this article as: Velloza J, Ngure K, Kiptinness C, Quame-Amaglo J, Thuo N, Dew K, Kimani M, Gakuo S, Unger JA, Kolko B, Baeten JM, Celum C, Mugo N, Heffron R. A clinic-based tablet application to support safer conception among HIV serodiscordant couples in Kenya: feasibility and acceptability study. mHealth 2019;5:4.


