
Development of a predictive nomogram for cause-specific mortality in surgically resected early-stage oesophageal cancer: a Surveillance, Epidemiology, and End Results (SEER) analysis
Introduction
Oesophageal cancer (EC) is predicted to be the seventh most commonly diagnosed cancer (an estimated 572,034 new cases) and the sixth leading cause of cancer-related death (an estimated 508,585 deaths) worldwide in 2018 (1). Only 5.0–11.1% of all patients with EC live five years or more after confirmed diagnosis, and even for patients with superficial EC (T1, located in mucosa or submucosa), almost 10.0–30.0% of this population will experience EC-related death within five years of surgery (1-5). Accordingly, the development of predictive models to precisely distinguish patients with unfavourable outcomes is critical to improving the survival rate of patients with early-stage EC, thereby increasing the overall survival rate of all EC patients. In addition, adequate and precise knowledge of prognostic outcomes can provide valuable information for aiding in decision making for multidisciplinary treatments, such as endoscopic treatment, postoperative adjuvant therapy, and intensive follow-up (3-5).
In addition, like with other malignancies, EC carries quite a high risk of competing cancer- and non-cancer-related deaths because almost 76.2% of patients with EC are over 60 years old, and nearly 50% of these patients are over 75 years old and have associated previous basic illness, such as cardiovascular and cerebrovascular diseases, respiratory diseases and metabolic diseases (6,7). Hence, the incidence of competing events increases with age, such as death from non-cancer diseases. Although many previous studies have explored prognostic factors for patients with early-stage EC using univariate and multivariate overall survival analyses, these competing risks have not been considered, which may make these independent prognostic factors untrustworthy (3,8-10). Indeed, for individualized cancer treatments, visualization of both cancer and non-cancer events contributing to the risk of death, based on competing risk models, is necessary (11,12). However, to date, a cause-specific analysis with an assessment of competing risks for surgically resected early-stage EC has not been reported.
Therefore, the aim of this study was to construct competing risk models and nomograms to evaluate cancer- and other cause-specific mortality in patients who underwent resection for pT1N0M0 EC by using the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database.
Methods
Study cohort
The study cohort for this research was downloaded from the SEER public-access database, which was released in June 3, 2018, using SEER*Stat (version 8.3.5) (13). First, all patients who were diagnosed with a primary site of oesophagus from 2010 to 2015, which was the period of adoption of the American Joint Committee on Cancer (AJCC) 7th edition TNM staging manual, were identified with the international classification of diseases for oncology, 3rd edition (ICD-O-3) site code (C15.0-C15.9), but no morphology code was limited (codes 8010-8015, 8020-8022, 8030-8035, 8041-8043, 8050-8089, 8140-8147, 8160-8162, 8170-8175, 8180-8231, 8250-8507, 8514-8551, 8571-8574, 8576, 8940-8982). However, only patients with pathological T0-1 and N0 and clinical M0 stage undergoing oesophagectomy were enrolled in this study. In addition, patients who received induction therapy, had other primary cancer(s) diagnosed after or before EC, were <18 years of age at diagnosis, with less than 3 months of survival, or with incomplete survival information (including follow-up months and cause of death) were excluded from this study. Finally, 1,144 patients with complete demographics, follow-up information, and clinicopathological data, including age, sex, race, year of diagnosis, insurance status, marital status, tumour length, pT subcategories, grade, histology, primary site, and regional nodes examination, were incorporated into our study (Figure 1).
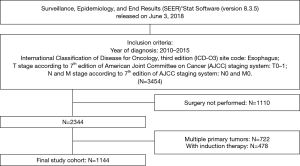
To evaluate the difference in mortality rates between different groups, these continuous variables—age, year of diagnosis and tumor length (cm)—were divided into categorical variable. Patients were divided into four age brackets (<50, 50–59, 60–69, >70 years) depended on age interval of 10 years. Tumour length (cm) was separated into three groups (<1, 1–5, ≥5 cm), and the cut-off values were determined by X-Tile software version 3.6.1 (Copyright Yale University 2003). Year of diagnosis was classified equally into three groups according to calendar year (2010–2011, 2012–2013, 2014–2015).
End points and competing risks
Cancer-specific mortality was defined as death due to EC, while death due to all non-cancer events was classified as other cause-specific mortality. Overall mortality included all deaths from cancer and non-cancer causes. However, in fact, only one end point can be recorded during the follow-up period. Therefore, cancer and non-cancer causes were regarded as two competing risk factors contributing to death.
Statistical analysis
The cumulative incidence rates of deaths for different competing events were calculated by competing risk analyses to show the cancer and non-cancer specific death probability, and only patients still alive at the date of last follow-up were documented as censored. In addition, the potential associations between these available perioperative variables from the SEER database and the risk of death from each cause were tested by using Fine and Grey’s regression analysis. These variables, if they were significant (P<0.05) in any univariate analysis group (cancer-specific death, death from other causes, and overall death), were retained in the multivariate regression analysis. All competing risk analyses were performed by using the R package cmprsk in R version 3.5.1 software (R Foundation for Statistical Computing, Vienna, Austria, http://www.r-project.org/).
To provide the oncologist with a quantifiable tool to predict the proportional subdistribution hazard of each cause-specific death for each patient with surgically resected early-stage (pT1N0M0) EC, competing risk nomograms were built based on the above multivariate regression analysis using R package rms. The discrimination performance of our competing risks nomograms was evaluated by bootstrapping validation with 200 resamples, and Harrell’s C-index was used to quantify the concordance between the predicted and observed probability of cause-specific death, which was achieved by the R package pec.
A two-sided P value less than 0.05 was regarded as a significant difference in all statistical tests.
Results
Patient characteristics
According to the above inclusion and exclusion criteria, a total of 1,144 patients (median age: 68 years, range: 30–87 years) diagnosed with pathological T1N0M0 EC from 2010 to 2015 in the National Cancer Institute’s SEER database were retained in the final analysis. The perioperative baseline characteristics of these patients are summarized in Table 1. Of these patients, the majority were elderly (≥60 years, 835/1,144, 73%), male (945/1,144, 82.6%), White race (1,042/1,144, 91.1%), and married (707/1,144, 61.8%). The lower third of the oesophagus was the most common site of primary EC (832/1,144, 72.8%) in patients who underwent oesophagectomy, followed by the middle third (124/1,144, 10.8%). However, other sites of primary EC recorded in the database, such as the upper third of the oesophagus, overlapping lesions of the oesophagus, etc., were only present in dozens of patients (the proportion was all less than 5%). In addition, 97.3% of patients (1,109/1,144) received at least one lymph node resection during the surgical operation (mean: 19.94, range: 1–99). In postoperative pathological examination, the majority of patients were diagnosed with adenocarcinoma (87.4%), had a tumour length less than 5 cm but more than or equal to 1 cm (72.4%) and were in the pT1a subcategory (56.8%). Information about the histological grade was available for 858 patients (75%), and the constituent ratios of grade I, II, III, and IV were 16.3%, 39.3%, 18.3%, and 1.1%, respectively.
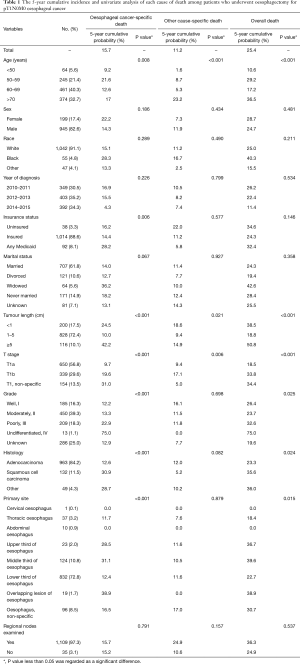
Full table
Overall, EC-specific and other cause-specific death
For patients with pT1N0M0 EC in this cohort, the median follow-up was 27.0 months (range, 0.5 to 71.0 months). A total of 291 deaths were observed during the follow-up period; however, approximately half (128 cases) of the deaths were due to competing risk events. The 5-year cumulative incidence of overall, EC-specific, other cause-specific mortality was 25.4% [95% confidence interval (CI), 21.7–29.1%], 15.7% (95% CI, 12.6–18.8%), and 11.2% (95% CI, 7.6–13.4%), respectively.
In univariate analysis, the characteristics of age, tumour length and pT1 subcategory were significantly related to the cumulative incidences of EC-specific, other cause-specific and overall death (all P<0.05), and the characteristics of histology, histological grade and primary tumour site were also significantly associated with EC-specific and overall mortality, but not with other cause-specific mortality. After the proportional subdistribution hazard analysis was performed by using the forward method, the following predictors were identified to conduct forecasting models for each outcome: age, tumour length, pT1 subcategory, histology, histological grade, primary tumour site for EC-specific death (C-index, 0.663, Table 2); age, tumour length, pT1 subcategory for other cause-specific death (C-index, 0.699, Table 2); and age, tumour length, pT1 subcategory for overall death (C-index, 0.578, Table 2). The 5-year cumulative probability of EC- and other cause-specific death by age, tumour length, and pT1 subcategory is showed in Figure 2, as determined by using the cumulative incidence function (CIF).
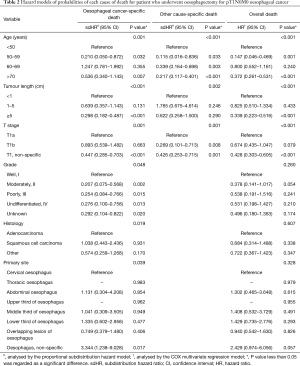
Full table

Predictive nomogram for cause-specific death
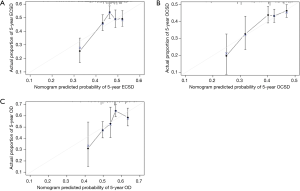
To predict the 1-, 3- and 5-year cumulative incidence of cause-specific death for patients with pT1N0M0 EC, these independent risk predictors identified by the proportional subdistribution hazard approach were used to construct a predictive nomogram based on Fine and Grey’s model (Figure 3). The C-index for the overall, EC- and other cause-specific mortality models was 0.578, 0.663 and 0.699, respectively, which showed that the models have a relative good discriminative ability. The calibration plots for the 5-year cumulative incidence of cause-specific death with the CI are presented in Figure 4, and the plots of predicted mortality for the bootstrap resampling group closely matched the ideal reference (45°) line, which indicates that the nomograms were well calibrated.
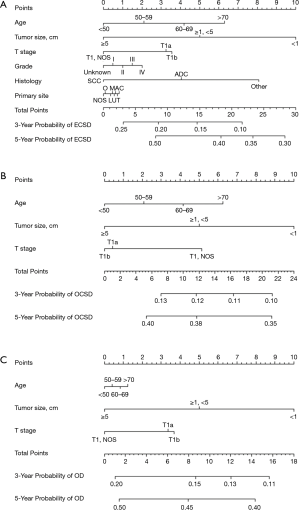
Discussion
First, this study identified cause-related risk factors to predict the specific cause of death for each patient diagnosed with pT1N0M0 EC in the SEER database between 2010 and 2015. Further, the independent cause-related risk factors identified by multivariate analysis were used to develop the quantifiable nomogram, which could predict the proportional subdistribution hazard of each cause-specific death.
With increasing age, the age-related comorbidities increased but physiological functions weakened; thus, advancing age may be the most representative of all competing events (7,14). In previous studies on early-stage oesophageal squamous cell carcinoma (ESCC) and adenocarcinoma (EAC), multivariate survival analyses indicated that advancing age was a strong prognostic factor of overall survival (9,15). Subsequently, Tang et al. performed a SEER-based study and identified age as an independent predictor to estimate cancer-specific survival of patients initially diagnosed with metastatic EC (advanced stage) (16). Similar to the results reported by Wu et al., advancing age had a negative impact on overall mortality, but not on cancer-specific mortality, of patients with EAC (regardless of the clinical-pathological stage), which was also the first competing risk in the analysis of EC. In the current study, we observed that the higher incidence of surgically resected early-stage EC-specific death was diminished in elderly patients (>70 years at diagnosis) and enhanced in younger patients (<60 years at diagnosis), consistent with results from Berry et al. (9). However, the obviously negative prognostic value of advancing age was demonstrated for other cause-specific mortality. Thus, the risk of death of the elderly, early-stage EC patients who died of cancer became closely equivalent to those who died of other causes. Thus, for elderly patients diagnosed with early-stage EC, the option of oesophagectomy should be fully considered with systemic conditions and comorbidities, and better postoperative basic life support should be as important as antitumour treatment to avoid excessive death from other causes.
In 2004, it was already reported that pT1 EC could be subcategorized into pT1a and pT1b according to whether tumour cells invaded the submucosa (17). However, the pT1 subcategorization of the oesophagus and oesophagogastric junction on the basis of survival differences to subdivide the stage I grouping was not added to the AJCC Cancer Staging Manual until 2010 (2). In this study, after Gary’s test and proportional subdistribution hazard analysis, we also found that patients with later pT1 substage (from pT1a, pT1b to pT1) had higher EC-specific mortality and overall mortality but lower other cause-specific mortality. Therefore, patients with earlier stage pT1 EC treated with oesophagectomy may have died from other causes before dying of cancer recurrence or metastasis. However, in a previous propensity score-matched study, patients with T1N0 EC who underwent local therapy had similar overall survival but improved EC-specific survival compared with those who underwent oesophagectomy, which meant that local therapy may reduce procedure-related death (9). In addition, Matsumoto and colleagues performed a single centre retrospective study and found that the prognosis following oesophagectomy was not better than that of chemoradiotherapy in elderly patients with stage I EC (18). Accordingly, the assessment of surgical tolerance among patients with pT1a stage EC who have potentially life-limiting medical conditions or comorbidities was critical, and palliative chemoradiotherapy or local therapy may be considered as treatment options for this population (4,9,18).
As we all know, patients diagnosed with more early-stage cancer had the chance to achieve longer survival, but the risk of dying from non-cancer causes increased. This phenomenon has been demonstrated and reported in stage I non-small-cell lung cancer, localized renal cell carcinoma, thyroid cancer, and non-metastatic malignant melanoma (11,12,19-21). However, a similar study on early-stage EC is still needed. In our competing risk analysis on patients with surgically resected early-stage (pT1N0M0) EC, a total of 291 patients died in the five-year follow-up period, and almost half of these patients died from non-cancer causes (128 patients). Subsequently, to numerically predict the probability of cause-specific death in surgically resected early-stage EC, the independent predictors identified by the proportional subdistribution hazard approach were used to develop a competing risk nomogram; to our knowledge, this is first time such a nomogram has been developed in EC. Our nomogram revealed a relative good discriminative ability and good calibration for both EC-specific death and other cause-specific death. Although our study was performed on data from the SEER database, the data in this national database were collected from various locations and sources throughout the United States (U.S.) and the population tends to have a higher proportion of foreign-born persons (17.9%) than of Americans (13.2%); thus, the competing risk nomogram obtained from SEER analysis had good applicability for prognostic judgement in different countries and areas (13). In addition, all variables enrolled in each cause-specific model were common and easily available in clinical practice. Therefore, during clinical decision making and patient-clinician communication, clinicians could apply the nomogram to make a rapid and precise prognosis judgement that relied only on medical records and postoperative pathology.
Although this large population-based cohort study that, for the first time, uses competing risk regression analysis in surgically resected early-stage EC has the greatest advantage, undeniably, several limitations should also be noted. First and foremost, some important factors related to prognosis of EC, such as history of alcohol use and smoking, cardio-pulmonary function, operation type, postoperative complications, tumour markers, genetic information, etc., were not documented in the SEER database; the recurrence time and site closely associated with EC-specific death were also unavailable (1,3,9,15,22). Second, because of the lack of pT1 staging before 2010 in the SEER database, patients enrolled in this retrospective study were selected from 2010 to 2015, so the follow-up time was relatively short. In addition, much progress in medical registration subsystems and follow-up strategies has occurred during the past two decades; thus, heterogeneity of the documented data sources was inevitable. Third, bootstrap resampling was selected as a cross-validation method to assess the predictive ability of our nomograms. Although each individual from the original data had an equal chance of being resampled, each random process could lead to an uneven calibration plot. Moreover, while our nomograms showed good applicability in internal validation, external applicability validated in another patient population could not be guaranteed. On the whole, our nomograms could be easily used in clinical practice without any complex calculations, and they graphically provided some references for prognosis judging and clinical decision making during patient counselling. More importantly, our study confirmed the feasibility of a nomogram in generating a numerical probability of cause-specific death in patients with early-stage EC and supplied a direction for future studies based on multicentre, large-scale cohorts with adequate follow-up time.
Conclusions
We carried out the first competing risk analysis for patients with surgically resected early-stage EC using the SEER database. Based on independent predictors identified by the final proportional subdistribution hazard analysis, a competing risks nomogram was developed to predict the proportional of risk of each cause of death. Our nomograms demonstrated good performance for risk stratification in the internal validation, but further external validation is needed to determine whether the nomogram can be applied to a wider population.
Acknowledgments
Read at the 99th Annual Meeting of the American Association for Thoracic Surgery, Toronto, Canada, May 4th-May 7th, 2019 (https://www.aats.org/aatsimis/AATSWeb/Association/Meetings/Annual_Meeting/99th_Annual_Meeting/AATS_99th_Annual_Meeting_Abstracts/2019-a-399-AATS.ASPX).
Funding: The preparation, English editing and publication of this manuscript were supported by the National Key Research and Development Plan (No. 2017YFC1308704), and the Ministry of Science and Technology of the People’s Republic of China.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jtd.2020.03.25). The authors have no conflict of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study based on the National Cancer Institute’s SEER database was approved by the institutional review board at Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (NCC201802006).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Yu X, Wen Y, Lin Y, et al. The value of preoperative Glasgow Prognostic Score and the C-Reactive Protein to Albumin Ratio as prognostic factors for long-term survival in pathological T1N0 esophageal squamous cell carcinoma. J Cancer 2018;9:807-15. [Crossref] [PubMed]
- Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus 2019;16:1-24.
- Shen W, Shen Y, Tan L, et al. A nomogram for predicting lymph node metastasis in surgically resected T1 esophageal squamous cell carcinoma. J Thorac Dis 2018;10:4178-85. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Janssen-Heijnen ML, Houterman S, Lemmens VE, et al. Prognostic impact of increasing age and co-morbidity in cancer patients: a population-based approach. Crit Rev Oncol Hematol 2005;55:231-40. [Crossref] [PubMed]
- Teramoto H, Koike M, Tanaka C, et al. Tumor budding as a useful prognostic marker in T1-stage squamous cell carcinoma of the esophagus. J Surg Oncol 2013;108:42-6. [Crossref] [PubMed]
- Berry MF, Zeyer-Brunner J, Castleberry AW, et al. Treatment modalities for T1N0 esophageal cancers: a comparative analysis of local therapy versus surgical resection. J Thorac Oncol 2013;8:796-802. [Crossref] [PubMed]
- Tanaka T, Matono S, Mori N, et al. T1 squamous cell carcinoma of the esophagus: long-term outcomes and prognostic factors after esophagectomy. Ann Surg Oncol 2014;21:932-8. [Crossref] [PubMed]
- Zhou H, Zhang Y, Qiu Z, et al. Nomogram to Predict Cause-Specific Mortality in Patients With Surgically Resected Stage I Non-Small-Cell Lung Cancer: A Competing Risk Analysis. Clin Lung Cancer 2018;19:e195-e203. [Crossref] [PubMed]
- Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol 2017;35:281-90. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program public-use data (1973-2015). National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, Released April 2018, Based on the November 2017 Submission. Available online: . Accessed June. 3 2018.http://www.seer.cancer.gov
- Carmona R, Zakeri K, Green G, et al. Improved Method to Stratify Elderly Patients With Cancer at Risk for Competing Events. J Clin Oncol 2016;34:1270-7. [Crossref] [PubMed]
- Yu X, Zhang R, Yang T, et al. Alpha-l-fucosidase: a novel serum biomarker to predict prognosis in early stage esophageal squamous cell carcinoma. J Thorac Dis 2019;11:3980-90. [Crossref] [PubMed]
- Tang X, Zhou X, Li Y, et al. A Novel Nomogram and Risk Classification System Predicting the Cancer-Specific Survival of Patients with Initially Diagnosed Metastatic Esophageal Cancer: A SEER-Based Study. Ann Surg Oncol 2019;26:321-8. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Rybicki LA, et al. Refining esophageal cancer staging. J Thorac Cardiovasc Surg 2003;125:1103-13. [Crossref] [PubMed]
- Matsumoto Y, Kimura K, Zhou Q, et al. Treatments and outcomes of older patients with esophageal cancer: Comparison with younger patients. Mol Clin Oncol 2019;11:383-9. [PubMed]
- Kutikov A, Egleston BL, Wong YN, et al. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol 2010;28:311-7. [Crossref] [PubMed]
- Yang L, Shen W, Sakamoto N. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol 2013;31:468-74. [Crossref] [PubMed]
- Shen W, Sakamoto N, Yang L. Melanoma-specific mortality and competing mortality in patients with non-metastatic malignant melanoma: a population-based analysis. BMC Cancer 2016;16:413. [Crossref] [PubMed]
- Wen YS, Huang C, Zhang X, et al. Impact of metabolic syndrome on the survival of Chinese patients with resectable esophageal squamous cell carcinoma. Dis Esophagus 2016;29:607-13. [Crossref] [PubMed]


