
(-)-Epigallocatechin-3-gallate induces interferon-λ2 expression to anti-influenza A virus in human bronchial epithelial cells (BEAS-2B) through p38 MAPK signaling pathway
Introduction
The influenza virus is a single-stranded negative-sense RNA virus belonging to the Orthomyxoviridae family, and can be divided into 4 types: of A, B, C, and D (1,2). Among these types, the influenza A virus (IAV) is the dominant pathogen. IAV is prone to antigenic variation, so it is seasonally prevalent every year. According to the latest report, influenza causes 290,000 to 650,000 deaths every year, and is a serious threat to human health (3). A universal influenza vaccine has not yet been developed although influenza type- and subtype-specific vaccine can be developed. Given this, considerable time and money have been expended to predict the prevalent strains and formulate effective vaccines (4). Among these measures, adamantanes and neuraminidase (NA) inhibitors are 2 classes of antiviral drugs that have been approved for treatment of influenza virus infections. However, influenza virus resistance to these drugs is a significant concern (5-7). There is therefore an urgent need for an effective strategy that can control the influenza virus.
Respiratory epithelium cells serve as the first line of defense against the influenza virus and culminate in the production of interferon (IFN), a key molecule in the antiviral innate immune response (8,9). However, the virulence factors of the influenza virus can antagonize the IFN immune response and promote influenza virus infection (10). Therefore, inducing IFN expression may be an effective strategy against the influenza virus. (-)-Epigallocatechin-3-gallate (EGCG) is the most abundant and bioactive catechin in green tea (11). Previous studies have revealed that EGCG exhibits affects against viral infection, including against the human immunodeficiency virus (HIV) (12), the herpes simplex virus (HSV) (13), the hepatitis B virus (HBV) (14), the hepatitis C virus (HCV) (15), and the Zika virus (16). It has been shown that EGCG enhances virus dsRNA or poly I:C-induced IFN-λ1 production and inhibits HCV replication (17,18). Although many studies have reported that EGCG has anti-influenza-virus effects (19), whether EGCG can induce IFN-λ expression in airway epithelial cells to suppress the influenza virus and related signaling pathways has not been elucidated at this stage.
In this study, we used the tracheal epithelial cells (BEAS-2B) as model to investigate EGCG’s anti-IAV effects and mechanisms. The results confirmed that EGCG can induce IFN-λ2 expression through p38 MAPK signaling pathway, but not through the ERK and JNK signaling pathways. It was also found that IFN-λ2 neutralizing antibody attenuated the role of EGCG inhibiting the expression of H1N1 nucleoprotein (NP) gene and protein. These findings provide some insight into the mechanisms behind EGCG’s anti-IAV (H1N1) activity.
Methods
Cell and virus culture
Human bronchial epithelial cells (BEAS-2B) and Madin-Darby canine kidney (MDCK) cells were purchased from the Kunming Cell Bank of the Chinese Academy of Sciences (Kunming, China). All the cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (FBS) (Gibco, Rockville, MD, USA) and 1% penicillin and streptomycin solution (HyClone, Logan, UT, USA) at 37 °C in an atmosphere of 5% CO2.
IAV samples (A/PR/8/34 H1N1, PR8) were purchased from the National Influenza Center, National Institute for Viral Disease Control and Prevention Chinese Center for Disease Control and Prevention (Beijing, China), and were amplified in the allantoic cavity of embryonated eggs for 48 h at 37 °C, and were stored after at −80 °C. Viral titers were determined by calculating the 50% tissue culture infectious dose (TCID50) per milliliter using the Reed–Muench method in MDCK cells.
Treat BEAS-2B cells
BEAS-2B cells were added into 96-well plates (1×104 cells/well) or 6-well plates (5×105 cells/well). After this, the BEAS-2B cells in 96-well plates were treated with different concentrations (i.e., 0, 6.25, 12.5, 25, 50, and 100 µg/mL) of EGCG (Solarbio, Beijing, China) for 48 h. The BEAS-2B cells in the 6-well plates were treated with different concentrations (i.e., 0, 6.25, 12.5, 25 and 50 µg/mL) of EGCG for 12 h; were treated with EGCG (50 µg/mL) for 0, 2, 4, 12, 24, and 48 h; or were incubated with 20 µM of p38 MAPK inhibitor (SB203580, Beyotime, ShangHai, China), 20 µM of ERK inhibitor (U1026, Beyotime, Shanghai, China), or 10 µM of JNK inhibitor (SP600125, Beyotime, Shanghai, China) dissolved in dimethyl sulfoxide (DMSO) for 1 h, with the supernatant being removed and mixed with EGCG(50µg/mL) for 4 h. The BEAS-2B cells in 6-well plates were treated with EGCG (50 µg/mL), EGCG (50 µg/mL) and IFN λ2 neutralizing antibody (0.5 µg/mL, MAB1587, R&D, Minneapolis MN, USA) or control antibody (0.5 µg/mL, MAB002, R&D, Minneapolis MN, USA) for 12 h. Next, the supernatant was removed, and cells were infected with IAV (H1N1) for 1 h at 37 °C. After the supernatant was removed, the cells were washed twice and recovered with DMEM to culture for 12 h.
Cell viability assessment
The BEAS-2B cells in 96-well plates were treated with different concentrations EGCG for 48 h. After that, 20 µL/well Counting Kit-8 (CCK8) (Dojindo, Kumamoto, Japan) was added into each well and incubated for 2 h at 37 °C. Then, the absorbance was read at 450 nm on a microplate reader (Bio-Tec Instruments, Burlington, VT, USA). The cell viability rate was calculated using the following formula: cell viability rate = [(ODEGCG (0-100µg/mL) – ODzeroing) / (ODEGCG (0µg/mL) – ODzeroing)] × 100%.
RNA isolation and quantitative real time polymerase chain reaction (qRT-PCR)
Total RNAs were isolated by using Total RNA extraction kit (Tian Gen, Beijing, China). The RNA purity and quantity were measured on NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Next, the PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Dalian, China) and total RNA template was used to synthesize the cDNA. The quantitative real-time polymerase chain reaction (qRT-PCR) was performed on CFX96 Real-Time PCR System (Bio-Rad, Hercules, CA, USA) by using the cDNA, SYBR Premix Ex Taq (Takara, Dalian, China), and the following pre-designed primers (GENEWIZ, Suzhou, China): IFN-λ2 forward: 5'-GGGCCTGTATCCAGCCTCAG-3' and reverse: 5'-GAGGAGGCGGAAGAGGTTGA-3'. NP forward: 5'-GACGATGCAACGGCTGGTCTG-3'and reverse: 5'-AGCATTGTTCCAACTCCTTT-3'. GAPDH forward: 5'-AGAAGGCTGGGGCTCATTTG-3' and reverse: 5'-AGGGGCCATCCACAGTCTTC-3'.
GAPDH was used as the reference gene for normalizing and determining the fold change in the expression of IFN-λ2 and NP mRNA, which was calculated using the 2(-Delta Delta Ct) method.
Enzyme-linked immunosorbent assay
After stimulation, the supernatants were gently collected to determine the protein concentrations of IFN-λ2 using commercially available ELISA kits (Elabscience, Wuhan, China) according to the manufacturer’s instructions.
Western blot analysis
Cells were washed with 37 °C PBS, mixed with RIPA Lysis Buffer for 10 min at 4 °C, and centrifuged to yield whole-cell lysates. The protein sample (20 µg) was separated by SDS polyacrylamide gels with electrophoresis (Bio-Rad, USA), and the gel was transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were blocked with blocking buffer for 2 h and then incubated overnight at 4 °C with the corresponding primary antibodies, including phospho-extracellular signal-regulated kinase (P-ERK) (1:1,000 dilution, #4370, CST, USA), ERK (1:1,000 dilution, #4695, CST, USA), phospho-c-Jun N-terminal kinase (P-JNK) (1:1,000 dilution, ab124956, Abcam, Cambridge, UK), JNK (1:1,000 dilution, ab179461, Abcam, Cambridge, UK), phospho-p38 mitogen-activated protein kinase (P-p38 MAPK) (1:1,000 dilution, #9211, CST, USA), p38 MAPK (1:1,000 dilution, #9212, CST, USA), NP (1:1,000 dilution, bs-4976R, Bioss, Beijing, China), and GAPDH (1:700, M00227-5, Boster, Wuhan, China). The membrane was washed with TBS-T and incubated with the HRP-goat anti-Rabbit IgG (1:5000 dilution, BA1054, Boster, Wuhan, China) as the secondary antibody and then developed in electrochemiluminescent western detection reagents (Millipore, Billerica, MA, USA).
Statistical analysis
Data are represented as means ± standard deviations (SD) of results from the 3 independent experiments with similar patterns. Significant differences between the indicated groups were determined by Student’s t-test and one-way analysis of variance (ANOVA) using GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA). For all experiments, P<0.05 was considered a statistically significant value.
Results
Cytotoxicity of EGCG on BEAS-2B cells
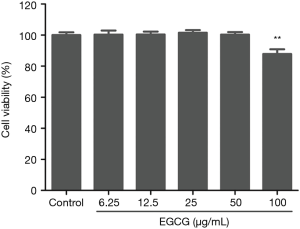
To confirm the cytotoxicity of EGCG on BEAS-2B cells, the BEAS-2B cells were treated with various concentrations (i.e., 12.5, 25, 50, and 100 µg/mL) of EGCG for 48 h, and then CCK-8 detection kit was used to detect the viability of cells. The result suggests treatment with EGCG at the concentration range of 0 to 50 µg/mL for 48 h elicits no cytotoxicity on BEAS-2B cells (Figure 1). Thus, this concentration range was applied in subsequent experiments.
EGCG induces IFN-λ2 expression in BEAS-2B cells
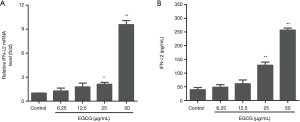
After detecting a concentration range in which EGCG is not cytotoxic to BEAS-2B cells, we next sought to determine the time and dose dependency that EGCG induces IFN-λ2 expression in BEAS-2B cells. Cells were treated with EGCG. The expression level of the IFN-λ2 mRNA was analyzed by qRT-PCR, and expression level of the IFN-λ2 protein was analyzed by ELISA. We found that both IFN-λ2 mRNA and protein levels were significantly up-regulated in BEAS-2B cells in a dose- and time-dependent manner after being treated with EGCG (Figures 2,3).
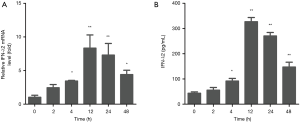
EGCG induces IFN-λ2 expression in BEAS-2B cells by p38 MAPK signaling pathway
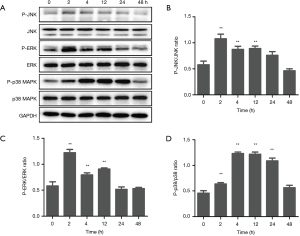
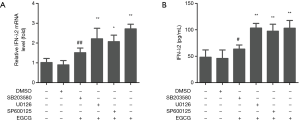
As the stimulation with 50 µg/mL of EGCG showed the strongest response in inducing the expression of IFN-λ2, we next selected 50 µg/mL EGCG to determine the signal pathways in which EGCG induces IFN-λ2 expression in BEAS-2B cells. BEAS-2B cells were treated with 50 µg/mL EGCG for 0, 2, 4, 12, 24, and 48 h, and we then determined the effect of EGCG on the activation of ERK, p38 MAPK, and JNK in BEAS-2B cells by western blot. As shown in Figure 4, the total ERK, p38 MAPK, and JNK levels had no effect after 50 µg/mL of EGCG treatment, but the phosphorylated (activation) ERK, p38 MAPK, and JNK levels were significantly up-regulated. The activation time of ERK and JNK occurred at 2 to 12 h, and that of p38 MAPK occurred at 2 to 24 h, following the treatment with EGCG. The result confirmed that EGCG can activate p38 MAPK, ERK, and JNK signaling pathways in BEAS-2B cells (Figure 4). Next, we tested the functions of p38 MAPK, ERK, and JNK signaling pathways in EGCG-induced IFN-λ2 mRNA and protein expression. The BEAS-2B cells were pretreated with 20 µM SB203580, 20 µM U0126, or 10 µM SP600125 for 1 h, after which time the 50 µg/mL of EGCG was mixed with the cells for 4 h. We found that pretreatment with SB203580 significantly inhibited EGCG-induced IFN-λ2 mRNA and protein expression, whereas pretreatment with U0126 and SP600125 did not show inhibition (Figure 5).
EGCG inhibits IAV H1N1 replication in BEAS-2B cells by inducing IFN-λ2 expression
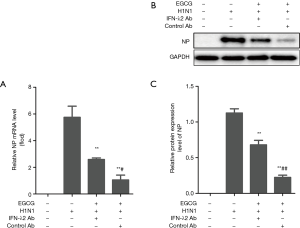
The results above confirmed that EGCG can induce the expression of IFN-λ2 in BEAS-2B cells. We next tested whether EGCG’s anti-IAV (H1N1) effect was related to the induced IFN-λ2 expression. The BEAS-2B cells were pretreated with 50 µg/mL EGCG, 50 µg/mL EGCG and 0.5 µg/mL IFN-λ2 neutralizing antibody, or 50 µg/mL EGCG and 0.5 µg/mL control antibody for 12 h, and then the cells were infected with 100 × TCID50 H1N1 for 1 h. The expression levels of the H1N1 NP gene and protein was analyzed by qRT-PCR and western blot, respectively. The results showed that EGCG significantly down-regulated the H1N1 NP gene and protein expression, while IFN-λ2 neutralizing antibody attenuated the effect of EGCG’s down-regulation of the H1N1 NP gene and protein expression (Figure 6).
Discussion
Green tea has been regarded as a health product for more than 2000 years and is a major source of polyphenol antioxidants (20,21). Green tea catechins, especially the main catechin found in green tea, epigallocate-chin-3-gallate (EGCG) have demonstrated anti-viral and anti-inflammatory properties (22). In the previous study, EGCG was found to inhibit influenza viral replication by damaging the physical properties of the viral envelope and partial inhibition of the NA surface glycoprotein (19). However, the immune regulation mechanisms of EGCG’ anti-IAV effects remain unclear. To our knowledge, this is the first study to demonstrate that EGCG up-regulates IFN-λ2 expression through p38 MAPK signaling pathway and inhibits IAV H1N1 replication in BEAS-2B cells.
The IFN-λ family, also known as type III IFN, includes the IFN-λ1, IFN-λ2, and IFN-λ3 members (22,23). In mice, only IFN-λ2 and IFN-λ3 are expressed (24). IFN-λ plays an important role in combating pathogen infection, has been shown to have protective effects against IAV infection, and to contribute to local anti-IAV innate immunity (25,26). More excitingly, the effect of IFN-λ against the IAV is better than that of type I IFN (27). However, the cost of directly using IFN-λ to treat influenza virus is high, and plant extracts have the advantage of low toxicity, easy availability, and being economical. In the previous study, the crude phenylethanoid glycosides isolated from Ligustrum purpurascens via inducing endogenous IFN-γ had an anti-viral effect against the IAV (28). In other studies, EGCG inhibited HCV replication by enhancing virus dsRNA or poly I:C-induced IFN-λ1 production (17,18). Consequently, these studies provided us with new ideas. In the present study, we used EGCG to treat BEAS-2Bs, with the results showing that EGCG significantly up-regulated IFN-λ2 mRNA and protein levels in BEAS-2B cells. Therefore, we confirmed that EGCG can induce IFN-λ2 production in BEAS-2B cells.
In addition, we also explored the mechanisms of EGCG-induced IFN λ2 expression in BEAS-2B cells. The MAPKs in mammals include c-Jun NH2-terminal kinase (JNK), p38 mitogen-activated protein kinase (p38 MAPK), and extracellular signal-regulated kinase (ERK). These enzymes are serine-threonine protein kinases that regulate various cellular activities including proliferation, differentiation, apoptosis or survival, inflammation, and innate immunity (29). EGCG treatment was able to induce NB4 cell apoptosis by increasing phosphorylated (p)-p38α MAPK expression (30). In addition, other studies have found that EGCG also regulates ERK and JNK signaling pathways (31,32). In the present study, we found that EGCG could significantly increase P-p38 MAPK, P-ERK, and P-JNK levels, and activate P38 MAPK, ERK, and JNK signaling pathways. Furthermore, p38 MAPK, ERK, or JNK inhibitor was used to inhibit signaling pathways: p38 MAPK inhibitor significantly decreased the EGCG-induced expression level of IFN-λ2 in BEAS-2B cells, while the ERK and JNK inhibitors had no effects. Our result is similar to the other study which also has demonstrated that IFN-λ1 was regulated by p38 MAPKα (33). Accordingly, our study has demonstrated that EGCG can induce the expression of IFN-λ2 through the p38 MAPK signaling pathway in BEAS-2B cells.
Besides, EGCG pretreatment significantly inhibited the expression levels of IAV H1N1 NP gene and protein in BEAS-2B cells. However, IFN-λ2 neutralizing antibody attenuated the effect of EGCG inhibiting the expression levels of IAV H1N1 NP gene and protein. Combining our new findings with other research results, EGCG not only inhibits influenza viral replication by damage to the physical properties of the viral envelope and partial inhibition of the NA surface glycoprotein (19) but also anti-IAV by inducing IFN-λ2 expression. A recent study demonstrated that EGCG could treat acute lung injury induced by swine influenza virus (H9N2) through 67LR/Tollip-downregulated TLR4 protein levels, decreased MPO activity and inflammatory cytokine levels (34). These findings suggest that EGCG can fight influenza viruses through a variety of mechanisms.
Conclusions
Our study demonstrated that EGCG can inhibit IAV H1N1 replication by inducing IFN-λ2 expression through p38 MAPK signaling pathway in BEAS-2B cells. Its effective regulation of IAV H1N1 replication implies that EGCG is a potential alternative treatment for the IAV.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hutchinson EC. Influenza Virus. Trends Microbiol 2018;26:809-10. [Crossref] [PubMed]
- Jiang WM, Wang SC, Peng C, et al. Identification of a potential novel type of influenza virus in Bovine in China. Virus Genes 2014;49:493-6. [Crossref] [PubMed]
- Yandrapalli S, Aronow WS, Frishman WH. Readmissions in adult patients following hospitalization for influenza: a nationwide cohort study. Ann Transl Med 2018;6:318. [Crossref] [PubMed]
- Nagesh PT, Mazhar H, Galvin HD, et al. Histone Deacetylase 2 Is a Component of Influenza A Virus-Induced Host Antiviral Response. Front Microbiol 2017;8:1315-26. [Crossref] [PubMed]
- Dong G, Peng C, Luo J, et al. Adamantane-Resistant Influenza A Viruses in the World (1902–2013): Frequency and Distribution of M2 Gene Mutations. Plos One 2015;10:e0119115. [Crossref] [PubMed]
- McKimm-Breschkin JL. Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance. Influenza Other Respir Viruses 2013;7:25-36. [Crossref] [PubMed]
- Hussain M, Galvin HD, Haw TY, et al. Drug resistance in influenza A virus: the epidemiology and management. Infect Drug Resist 2017;10:121-34. [Crossref] [PubMed]
- Baturcam E, Vollmer S, Schlüter H, et al. MEK inhibition drives anti-viral defence in RV but not RSV challenged human airway epithelial cells through AKT/p70S6K/4E-BP1 signalling. Cell Commun Signal 2019;17:78-96. [Crossref] [PubMed]
- Commins SP, Borish L, Steinke JW. Immunologic messenger molecules: Cytokines, interferons and chemokines. J Allergy Clin Immunol 2010;125:S53-72. [Crossref] [PubMed]
- Nogales A, Martinez-Sobrido L, Topham D, et al. Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins. Viruses 2018. [Crossref] [PubMed]
- Vázquez Cisneros LC, López-Uriarte P, López-Espinoza A, et al. Effects of green tea and its epigallocatechin (EGCG) content on body weight and fat massin humans: a systematic review. Nutr Hosp 2017;34:731-7. [PubMed]
- Castellano LM, Hammond RM, Holmes VM, et al. Epigallocatechin-3-gallate rapidly remodels PAP85-120, SEM1(45-107), and SEM2(49-107) seminal amyloid fibrils. Biol Open 2015;4:1206-12. [Crossref] [PubMed]
- Shivani NP, Sandra DA, Lee HL, et al. Inhibition of Herpes Simplex Virus-1 by the Modified Green Tea Polyphenol EGCG-Stearate. ABB 2018;9:679-90. [Crossref]
- Lai YH. Epigallocatechin gallate inhibits hepatitis B virus infection in human liver chimeric mice. BMC Complement Altern Med 2018;18:248. [Crossref] [PubMed]
- Calland N, Albecka A, Belouzard S, et al. (-)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 2012;55:720-9. [Crossref] [PubMed]
- Sharma N, Murali A, Singh SK, et al. Epigallocatechin gallate, an active green tea compound inhibits the Zika virus entry into host cells via binding the envelope protein. Int J Biol Macromol 2017;104:1046-54. [Crossref] [PubMed]
- Wang Y, Li J, Wang X, et al. (-)-Epigallocatechin-3-Gallate Enhances Hepatitis C Virus Double-Stranded RNA Intermediates-Triggered Innate Immune Responses in Hepatocytes. Sci Rep 2016;6:21595-606. [Crossref] [PubMed]
- Wang YZ, Li JL, Wang X, et al. (-)-Epigallocatechin-3-gallate enhances poly I:C-induced interferon-λ1 production and inhibits hepatitis C virus replication in hepatocytes. World J Gastroenterol 2017;23:5895-903. [Crossref] [PubMed]
- Ide K, Kawasaki Y, Kawakami K, et al. Anti-influenza Virus Effects of Catechins: A Molecular and Clinical Review. Curr Med Chem 2016;23:4773-83. [Crossref] [PubMed]
- Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: A literature review. Chin Med 2010;5:13-21. [Crossref] [PubMed]
- Hu J, Gu J, Xu F, et al. Epigallocatechin-3-gallate Attenuates Myocardial Reperfusion Injury in Rats Through Activation of PI3K/Akt Signaling Pathway. Sichuan Da Xue Xue Bao Yi Xue Ban 2016;47:305-9. [PubMed]
- Kotenko SV, Gallagher G, Baurin VV, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 2003;4:69-77. [Crossref] [PubMed]
- Sheppard P, Kindsvogel W, Xu W, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 2003;4:63-8. [Crossref] [PubMed]
- Lasfar A, Lewisantes A, Smirnov SV, et al. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res 2006;66:4468-77. [Crossref] [PubMed]
- Klinkhammer J, Schnepf D, Ye L, et al. IFN-λ prevents influenza virus spread from the upper airways to the lungs and limits virus transmission. Elife 2018. [Crossref] [PubMed]
- Kim S, Kim MJ, Kim CH, et al. The Superiority of IFN-lambda as a Therapeutic Candidate to Control Acute Influenza Viral Lung Infection. Am J Respir Cell Mol Biol 2017;56:202-12. [PubMed]
- Davidson S, Mccabe TM, Crotta S, et al. IFN-λ is a potent anti-influenza therapeutic without the inflammatory side effects of IFN-α treatment. EMBO Mol Med 2016;8:1099-112. [Crossref] [PubMed]
- Hu XP, Shao MM, Song X, et al. Anti-influenza virus effects of crude phenylethanoid glycosides isolated from ligustrum purpurascens via inducing endogenous interferon-γ. J Ethnopharmacol 2016;179:128-36. [Crossref] [PubMed]
- Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Arch Toxicol 2015;89:867-82. [Crossref] [PubMed]
- Gan L, Zhong L, Shan Z, et al. Epigallocatechin-3-gallate induces apoptosis in acute promyelocytic leukemia cells via a SHP-1-p38α MAPK-Bax cascade. Oncol Lett 2017;14:6314-20. [PubMed]
- Satonaka H, Ishida K, Takai M, et al. (-)-Epigallocatechin-3-gallate Down-regulates Doxorubicin-induced Overexpression of P-glycoprotein Through the Coordinate Inhibition of PI3K/Akt and MEK/ERK Signaling Pathways. Anticancer Res 2017;37:6071-7. [PubMed]
- Xiao X, Jiang K, Xu Y, et al. (-)-Epigallocatechin-3-gallate induces cell apoptosis in chronic myeloid leukaemia by regulating Bcr/Abl-mediated p38-MAPK/JNK and JAK2/STAT3/AKT signalling pathways. Clin Exp Pharmacol Physiol 2019;46:126-36. [Crossref] [PubMed]
- Jiang M, Osterlund P, Fagerlund R, et al. MAP kinase p38 regulates type III interferon (IFN-λ) gene expression in human monocyte-derived dendritic cells in response to RNA stimulation. J Leukoc Biol 2015;97:307-20. [Crossref] [PubMed]
- Xu MJ, Liu BJ, Wang CL, et al. Epigallocatechin-3-gallate inhibits TLR4 signaling through the 67-kDa laminin receptor and effectively alleviates acute lung injury induced by H9N2 swine influenza virus. Int Immunopharmacol 2017;52:24-33. [Crossref] [PubMed]


