
Prognostic significance of anaplastic lymphoma kinase rearrangement in patients with completely resected lung adenocarcinoma
Introduction
Lung cancer is the most commonly diagnosed cancer (11.6% of the total cases) and the leading cause of cancer death (18.4% of the total cancer deaths) (1). Two main types of lung cancer are small cell lung cancer (SCLC) (10–15%) and non-small cell lung cancer (NSCLC) (80–85%) (2). NSCLC is subdivided into adenocarcinoma, squamous cell carcinoma (SQCC) and large cell carcinoma. Adenocarcinomas include adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), invasive adenocarcinoma and variants of invasive adenocarcinoma. Both AIS and MIA are associated with good prognosis.
The patient with anaplastic lymphoma kinase (ALK) gene rearrangement, which is caused by the translocation or inversion of chromosome 2p, is an important patient subset of lung cancer. The prevalence of ALK positive patients has been reported to range from 3% to 7% in advanced NSCLC (3-6), and 2.3% to 8.6% in early stage NSCLC (7-14). ALK positivity is correlated with adenocarcinoma histology, particularly the solid and signet ring pattern; never or light/former smoking status; younger age; and wild type for EGFR or KRAS gene mutation (5,15-19).
ALK was first discovered in 1994 as a fusion oncogene with nucleophosmin (NPM) in a subset of anaplastic large-cell lymphomas (ALCLs) (20). However, it was not until 12 years ago that interest in ALK surged after the discovery of a novel ALK fusion—echinoderm microtubule-associated protein-like 4 (EML4)-ALK, a somatic gene rearrangement found in a small portion of Japanese lung cancers (21). EML4-ALK is formed by an inversion occurring on the short arm of chromosome 2 involving the genes encoding ALK (2p23) and EML4 (2p21) with variants 1, 2, and 3a/3b (22,23). The three major variants (v1: E13; A20, v2: E20; A20, and v3; E6; A20) account for more than 90% of lung cancers associated with EML4-ALK. In addition to EML4-ALK, several other ALK fusions have been reported, including TRK-fused gene (TFG)-ALK, kinesin family member 5B (KIF5B)-ALK and kinesin light chain 1 (KLC1)-ALK (15,24,25). At the cellular level, ALK regulates canonical signaling pathways that are shared with other receptor tyrosine kinases (RTK) including RAS-mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)-AKT, and JAK-STAT pathways (26). In ALK rearrangements, 5' end partners such as EML4 and NPM are fused to the intracellular tyrosine kinase domain of ALK. The domains in the partner proteins promote dimerization and oligomerization of the fusion proteins, inducing constitutive activation of the ALK kinase and its downstream signaling pathways. This leads to uncontrolled cellular proliferation and survival. The EML4-ALK fusion gene possesses powerful oncogenic activity, both in vivo and in vitro (21,27), which might result in poor prognosis of NSCLC. However, several published studies show the conflicting results about the prognostic value of ALK rearrangement in NSCLC (7-14,28-31). Tantraworasin (10), Paik (8), Fukui (29), and Ohba (12), demonstrated that ALK positivity was not correlated with prognosis. Conversely, five reports revealed that patients with ALK rearrangement NSCLC had a shorter DFS (7,9,13,14,28). In contrast, Blackhall et al. reported superior RFS and OS in patients with ALK positive early-stage NSCLC (11). Preclinical studies demonstrate that ALK-driven lung cancers are addicted to ALK and highly sensitive to ALK inhibition (27,32), indicating that ALK rearrangement is a predictive factor for the therapeutic effect of ALK inhibitors. Additionally, several ALK inhibitors are already approved for the first line treatment of advanced stage ALK-positive NSCLC due to their encouraging therapeutic effect (33-36). The prognostic value of ALK rearrangement will help guide management and formulate statistical assumptions in the design of future ALK inhibitor– based adjuvant clinical trials. However, the prognostic significance of ALK rearrangement remains unclear and further investigation is needed.
The major objectives of the present study are not only to compare the clinical outcomes of ALK-positive versus ALK-negative completely resected stage I-IIIA lung adenocarcinoma patients, but also to explore the correlation of ALK rearrangement with clinical characteristics.
Methods
Study population and data collection
In our study, 2,103 patients with pathological stage I–IIIA lung adenocarcinoma who underwent complete resection in Shanghai Chest Hospital between July 2013 and December 2014, with at least 4 years of follow-up were included in the study. The patients who received neoadjuvant chemotherapy or radiotherapy were excluded. The patients did not receive ALK-targeted therapy before tumor recurrence in our study cohort. Histological types of lung adenocarcinoma are determined according to 2015 WHO classification of lung adenocarcinoma. The predominant pattern was defined as the pattern with the largest percentage. Lung cancer pathologic staging of the patients was based on the 8th edition of the TNM classification. All patients’ clinicopathologic characteristics were collected from the medical recording system. This study was approved by Ethics Committee of Shanghai Chest Hospital Jiao Tong University.
Detection of ALK rearrangement
Immunohistochemistry (IHC) was performed for all patients on 5-µm thick formalin-fixed paraffin-embedded surgical specimens with the fully-automated Ventana IHC system using the D5F3 anti-ALK rabbit monoclonal primary antibody in a Bech-mark XT staining module (Ventana Medical Systems, Illkirch Graffenstaden, France). The ALK status was described by a binary scoring system, either ALK positive or ALK negative. The histopathologic types and ALK status were evaluated independently by two experienced pathologists of Shanghai Chest Hospital.
Clinical outcomes and statistical analysis
Clinical outcomes were presented by overall survival (OS), defined as the time interval from date of surgery to death from any cause; disease-free survival (DFS), defined as the time from date of surgery to disease recurrence or death from any cause. If recurrence or death was not observed, the censoring date was the last day of follow-up. Both OS and DFS were calculated in months.
Statistical analyses were performed using SPSS®, version 24.0 (SPSS Inc., Chicago, IL, USA). Comparison of clinical characteristics according to ALK status was performed using Mann-Whitney U tests (continuous variables) and chi-square tests or Fisher’s exact tests (categorical variables).
Association between time-to-event outcomes and ALK status is only explored in ALK matched cohort. For this cohort, survival was estimated by the Kaplan-Meier method and compared using the log-rank test. The median follow-up time was estimated using the reverse censoring method for OS. Cox proportional hazards regression analysis was used to calculate the hazard ratio (HR) and 95% confidence interval (CI). Multivariable Cox regression, with the backward elimination procedure (removal criterion of 10%), was used to choose the best model for DFS and OS, examining characteristics including sex, age at surgery, smoking status, pathologic tumor stage (pT stage), pathologic nodal stage (pN stage), adenocarcinoma subtypes and ALK status. In all analyses, two-tailed P<0.05 was considered statistically significant.
Propensity score matching (PSM)
PSM was used to control for confounding effects of known predictors for lung cancer progression or recurrence. PSM was carried out in stage IA, IB, IIA, IIB, IIIA cohort respectively to guarantee the exact balance of pathologic stage, which was considered to be the most important prognostic factor, between ALK positive and ALK negative patients in the matched cohort. Propensity scores for all patients were calculated by using a multiple logistic regression with the following covariates: age, sex, type of surgery, histological subtypes and pleura invasion status. In the ALK matched cohort, all 81 ALK positive patients were matched 1:1 with 81 ALK negative patients. The clinical characteristics baseline before and after PSM were shown in Table S1.
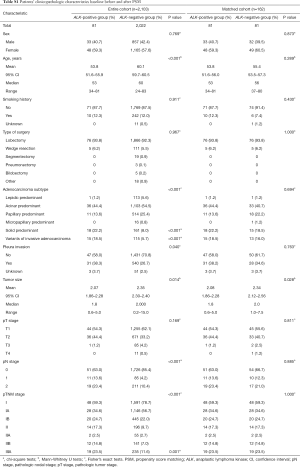
Full table
Follow up
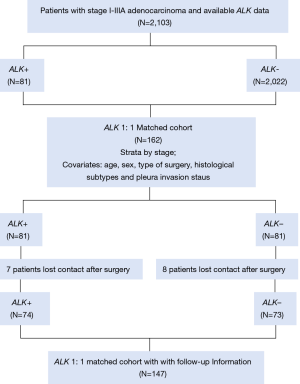
The follow-up data of the matched cohort were obtained by official contact with patients or their relatives by telephone or collected from hospital records. In the matched cohort of 162 patients, seven patients lost contact after surgery in the ALK positive group, and 8 patients in the ALK negative group. The workflow of the determination of the ALK status and the populations identified is depicted in Figure 1.
Routine examinations, such as a plain chest X-ray; computed tomography scan of the thorax, head, and abdomen; and ultrasound of neck and abdomen, were generally performed every 3 months for the first 2 years after surgery and every 6 months after that for 5 years. After 5 years, the patients were assessed annually. Bone scans were performed as clinically indicated on the basis of bone pain. Positron emission tomography and bronchoscopy with biopsy were performed at the treating physician’s discretion.
The follow-up period was completed in December 2018 or to the death date of patients. The median follow-up was 55.3 months (interquartile range, 51.6 to 60.2 months).
Results
Clinicopathologic characteristics of ALK positive patients
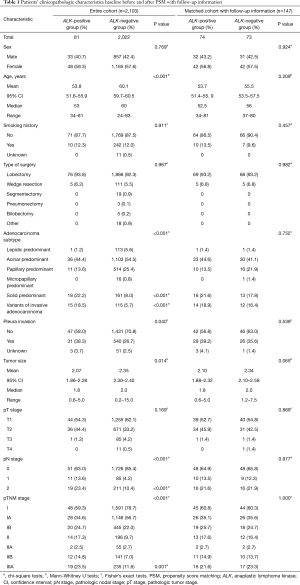
The clinicopathologic characteristics of 2,103 completely-resected stage I–IIIA lung adenocarcinoma patients are shown in Table 1. Eighty-one (3.9%) of the 2,103 patients were ALK positive. Eight hundred and ninety patients (42.3%) were male, and 1,213 (57.7%) were female; age (year) at surgery ranges from 24 to 83. A total of 1,840 (87.5%) were never-smokers, 252 (12.0%) were smokers, and 11 (0.5%) patients’ smoking status were unknown. Tumor size (cm) ranged from 0.2 to 15.0. The pathologic stage was stage I in 1,639 patients (77.9%), stage II in 210 (10.0%), and stage IIIA in 254 (12.1%). Invasive adenocarcinoma is the only histopathologic subtypes of the whole cohort with lepidic predominant, acinar predominant, papillary predominant, micropapillary predominant, and solid predominant subtypes present in 114 (5.4%), 1,139 (54.2%), 525 (25.0%), 16 (0.8%), and 179 (8.5%) patients, respectively, and variants of invasive adenocarcinoma in 130 patients (6.2%). Pleura invasion occurred in 571 (27.2%) patients.
Full table
ALK positivity was significantly associated with younger age (median age, 53 years in the ALK positive group vs. 60 years in the ALK negative group; P<0.001), solid predominant adenocarcinoma (P<0.001), variants of invasive adenocarcinoma (P<0.001), higher frequency of pleura invasion (P=0.040), smaller tumor size(median size, 1.8 cm in the ALK positive group vs. 2.0 cm in the ALK negative group; P=0.014), mediastinal lymph node involvement (N2; P<0.001), later pathologic stage (IIIA; P=0.001) (Table 1).
However, there were no significant associations between ALK status and other factors such as sex (P=0.769), smoking status (P=0.911), and pathologic tumor stage (P=0.169) (Table 1).
Clinicopathologic characteristics baseline data before and after weighting
Table 1 also shows the clinicopathologic characteristics baseline of the patients after PSM with follow-up information. A total of 81 (3.9%) and 2,022 (96.1%) patients were assigned to the ALK positive group and ALK negative group, respectively. Before PSM, differences were observed in terms of age (P<0.001), adenocarcinoma subtypes (P<0.001), pleura invasion status (P=0.040), tumor size (P=0.014), pN stage (P<0.001), pTNM stage (P<0.001) (Table 1); after PSM, the results were similar between the two groups (P>0.05) except for tumor size (P=0.028) (Table S1), and after the follow-up with 7 patients in the ALK positive group and 8 patients in the ALK negative group losing contact after surgery, the results were still similar between the two groups in the remaining 147 patients even for tumor size (P>0.05; Table 1).
Prognostic value of the ALK rearrangement in completely-resected stage I–IIIA lung adenocarcinoma
We next evaluated the associations between ALK rearrangement and prognosis in the 147 completely-resected stage I–IIIA lung adenocarcinoma patients. At the time of analysis, the median follow-up time was 55.3 months (interquartile range, 51.6 to 60.2 months). At last follow-up evaluation, a total of 18 (12.2%) of 147 patients died and all deaths were tumor-related, with a 4-year OS rate of 90.5%. The median OS time is not yet reached. A total of 55 (37.4%) of 147 patients experienced a DFS event, with a 4-year DFS rate of 64.0%. The median DFS time was also not yet reached.
The 4-year DFS rates were 66.2% in the ALK positive group and 61.9% in the ALK negative group. The 4-year OS rates in ALK positive and negative group were 94.6% and 86.3%, respectively. The median DFS and median OS of both ALK positive group and ALK negative group were not yet reached. The log-rank test showed that ALK positivity was not associated with better DFS or OS (DFS, P=0.289; OS, P=0.549; Figure 2). We further analyzed the associations between ALK positivity and prognosis by pathologic stage. The median OS of both ALK positive group and ALK negative group were not yet reached in each stage. The median DFS of both two groups was not reached in stage I. The median DFS of ALK positive group and ALK negative group is not reached and 54.8 months respectively in stage II. The median DFS of ALK positive group and ALK negative group is 35.2 and 15.9 months respectively in stage IIIA. The log-rank test still showed no significantly difference of DFS and OS between ALK positive group and ALK negative group in each stage (I: DFS, P=0.535; OS, P=0.565; II: DFS, P=0.903; OS, P=0.338; IIIA: DFS, P=0.138; OS, P=0.068; respectively).
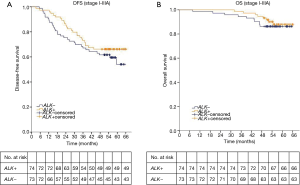
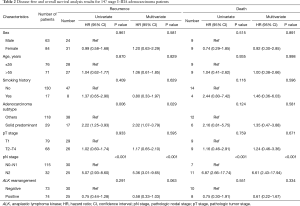
A univariate analysis showed that disease free survival was significantly shorter in patients with high lymph node status (N2) (HR: 5.07, 95% CI: 2.93–8.60, P<0.001; Table 2) or solid predominant adenocarcinoma subtype (HR: 2.22, 95% CI: 1.25–3.93, P=0.006; Table 2). And lymph node status was the only prognostic factor of OS (HR: 6.87, 95% CI: 2.66–17.74, P<0.001; Table 2). ALK positivity was not associated with better DFS or OS (HR, 0.75; 95% CI: 0.44–1.28; P=0.291; HR: 0.75, 95% CI: 0.30–1.91, P=0.551, respectively; Table 2). A multivariate analysis using a Cox proportional hazards model compared survival between ALK positive and ALK negative patients. After adjusting for sex, age, smoking history, adenocarcinoma subtypes, pathologic nodal staging, tumor staging and ALK rearrangement status, the variables that remained significantly associated with decreased DFS were mediastinal lymph node involvement (HR: 5.36, 95% CI: 3.01–9.65, P<0.001; Table 2) and solid predominant adenocarcinoma subtype (HR, 2.02; 95% CI: 1.07–3.79; P=0.029; Table 2). ALK positivity was not associated with DFS (HR, 0.58; 95% CI: 0.33–1.03, P=0.063; Table 2) or OS (HR, 0.61; 95% CI: 0.22–1.67, P=0.334; Table 2). These results suggested that ALK rearrangement may not be a prognostic factor in completely resected stage I–IIIA lung adenocarcinoma.
Full table
The association of ALK positivity with the initial recurrence site
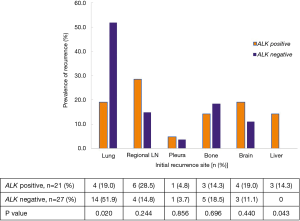
There were 55 patients with recurrent tumor in the matched cohort with follow up information. And 7 patients detected multiple recurrence sites at the same time and it was hard to find out what was really the initial site of these patients. In this case, we analyzed the association of ALK positivity with initial recurrence site in the remaining 48 patients with mono recurrence site. As shown in Figure 3, we found that there was an association between ALK status and liver and lung recurrence, more patients experienced liver recurrence and less experienced lung recurrence in ALK positive group than in ALK negative group [14.3% (3/21) vs. 0% (0/27), P=0.043; 19.0% (4/21) vs. 51.9% (14/27), P=0.020, respectively, Figure 3]. There were no differences of other initial recurrence sites including regional lymph nodes, pleura, bone and brain between the two groups.
Discussion
The prevalence of ALK positive patients was 3.9% in our study, consistent with the previous reports looking at unselected populations with mostly advanced adenocarcinoma (3-6). Several studies showed a higher prevalence of ALK positivity in younger patients, light smokers or never-smokers, females (15,16,28,30,37), and solid predominant adenocarcinoma subtype (14,38,39). In this study, we also found ALK rearrangements were detected more frequently in younger age patients and solid predominant adenocarcinoma subtype. However, ALK positivity showed no association with sex or smoking status, in accord with the results reported in other two studies (10,14). Although several previous studies showed that ALK rearrangement was not related to pleural invasion, we found ALK positive patients tend to have pleural invasion more frequently compared with ALK negative patients. A recent meta-analysis concluded that ALK rearrangement was more common in higher pathologic stages (40), which is in line with our results (IIIA, P=0.001). Furthermore, a previous study found that ALK positive lung cancer showed earlier tumor stage (T1) (P=0.02) (8), whereas it tended to harbor lymph node metastasis in adenocarcinoma (P=0.09), which is also consistent with our results. We revealed that ALK positive patients were more likely to have smaller tumor size (P=0.014) and mediastinal lymph node involvement (P<0.001). However, no significant difference of pathologic tumor stage (P=0.169) between ALK positive and negative group was observed in our study.
The prognostic value of ALK rearrangement in early stage NSCLC is controversial. Tantraworasin (10), Paik (8), Fukui (29), and Ohba (12), demonstrated that ALK positivity was not correlated with prognosis, which is consistent with our results. Conversely, five reports revealed that patients with ALK rearrangement NSCLC had a shorter DFS after adjusting for main prognostic clinical factors (7,9,13,14,28), and two studies showed that ALK positivity was not associated with OS (9,13), while other two studies concluded that ALK positive patients had inferior OS (7,14). In contrast, Blackhall et al. reported superior RFS and OS in patients with ALK positive early-stage NSCLC (11). To our knowledge, our study is one of the largest data set to report on the outcome of ALK positive patients with stage I to IIIA resected lung adenocarcinoma. Since the significant discrepancies of age, adenocarcinoma subtypes, pTNM stage etc. between the ALK positive group and ALK negative group in the entire cohort, we used PSM method to control the confounding effects of these known prognostic factors for lung cancer recurrence before we compared the prognostic impact of ALK rearrangement. And this is the first report using PSM to reveal that ALK positivity is not associated with DFS or OS. It indicates that ALK rearrangement is not an independent prognostic factor in stage I to IIIA completely resected lung adenocarcinoma patients.
In patients with advanced NSCLC, Shaw et al. found that ALK FISH-positive patients seemed to have similar survival to that of the general population of wild-type patients lacking either ALK rearrangement or EGFR mutation (41), whereas in a report in patients free of Crizotinib with wild-type EGFR lung adenocarcinoma, ALK rearrangement was associated with longer OS (42). As to patients with early stage NSCLC, Chaft et al. found that adjusted for stage ALK rearrangement NSCLC was associated with worse RFS compared to EGFR-mutant, but not when compared to KRAS-mutant (31), In our study, among 73 ALK negative patients in the matched cohort, there were 19 patients with EGFR-mutation,13 with EGFR wild-type and 41 with unknown EGFR mutational status. Adjusted for main prognostic clinical factors, ALK positive patients showed better DFS compared to both EGFR-mutant and EGFR wild-type patients (HR 0.29, 95% CI: 0.14–0.61, P=0.001; HR 0.27, 95% CI: 0.12–0.63, P=0.002, respectively). However, there was no significant difference of DFS between ALK positive patients and EGFR status unknown patients (HR 1.42, 95% CI: 0.61–3.33, P=0.421). These results might attribute to that EGFR status was not regularly tested in our study cohort after surgery. In this case, ALK negative patients who had already experienced recurrence were more likely to undergo the test for EGFR mutation to find out whether they could be treated with EGFR-tyrosine kinase inhibitors (TKI). This might result in significantly higher prevalence of recurrence in patients with clear EGFR status than ALK positive or unknown EGFR status patients in our study cohort. Therefore, it is plausible that the prognostic significance of ALK will alter relative to the EGFR mutational status of ALK-negative patients.
Furthermore, we found that mediastinal lymph node involvement (N2) and solid predominant adenocarcinoma subtype were independent prognostic factors of DFS, while mediastinal lymph node involvement (N2) was the independent prognostic factor of OS. Previous studies have shown that patients with solid predominant adenocarcinoma have poor prognosis (43-45), which is consistent with our results. Notably, a higher prevalence of ALK positivity in mediastinal lymph node involvement (P<0.001) and solid predominant adenocarcinoma subtype (P<0.001) was found in our study. When the balance was achieved for these two factors in the matched cohort, there were no significant differences of DFS and OS between ALK positive group and ALK negative group. This result indicated that ALK rearrangement might have an indirect impact on prognosis through its unique biologic features with early nodal metastasis and solid predominant adenocarcinoma subtype. However, when these factors were adjusted using PSM, we found ALK rearrangement was not an independent prognostic factor.
Yang et al. reported that ALK-positive tumors might have an increased risk of brain and liver metastases compared with ALK-negative disease in late stage (28). In our study, we found that more patients experienced liver recurrence and less experienced lung recurrence in ALK positive group than in ALK negative group. This result indicated that there was an association between ALK status and liver and lung recurrence. But the risk of brain metastases was similar in two groups.
Nevertheless, our study has several limitations. Firstly, we did not analyze the survival of entire cohort and the method PSM has its intrinsic limitation including that there may be other prognostic factors not covered in our regression model. Secondly, fifteen patients were lost to follow-up, although the clinicopathologic characteristics were still balanced between the two groups in the remaining patients. Thirdly, the EGFR or KRAS status were unknown in more than half of ALK negative patients, which made it challenging to analyze the prognostic impact of these genomic subsets. Fourthly, post-recurrence therapy information was lacking in our study and this might affect the OS of patients who experienced recurrence. In addition, since the majority of the patients in our study cohorts were woman and non-smokers, which is not typical of a non-East Asian population with lung cancer, the applicability of this study’s results may be limited in North American/European population.
Conclusions
ALK rearrangement was not an independent prognostic factor in stage I–IIIA lung adenocarcinoma patients, but it significantly correlated with younger age, solid predominant adenocarcinoma, higher frequency of pleura invasion, smaller tumor size, mediastinal lymph node involvement and later pathologic stage. In addition, there was an association between ALK status and liver and lung recurrence, more patients experienced liver recurrence and less experienced lung recurrence with ALK positive tumors than with ALK negative tumors.
Acknowledgments
Funding: This work was funded by the National Key R&D Program of China (2016YFC1303300 to S Lu), the National Natural Science Foundation of China (81672272 to S Lu), the Shanghai Municipal Science & Technology Commission Research Project (17431906103 to S Lu), the Shanghai Shenkang Action Plan (16CR3005A to S Lu), and the National Science and Technology Major Projects of New Drug Creation (2018ZX09301014-001-003 to S Lu), Shanghai Economic and Information Commission for oncology data platforms (201602010).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by Ethics Committee of Shanghai Chest Hospital. Written informed consent was obtained from all individual participants included in this study. And this study was conducted in accordance with the Declaration of Helsinki.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Molina JR, Yang PG, Cassivi SD, et al. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Tsao AS, Scagliotti GV, Bunn PA, et al. Scientific Advances in Lung Cancer 2015. J Thorac Oncol 2016;11:613-38. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic Lymphoma Kinase Inhibition in Non-Small-Cell Lung Cancer. New Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Vidal J, Clave S, de Muga S, et al. Assessment of ALK Status by FISH on 1000 Spanish Non-Small Cell Lung Cancer Patients. J Thorac Oncol 2014;9:1816-20. [Crossref] [PubMed]
- Kim HR, Shim HS, Chung JH, et al. Distinct clinical features and outcomes in never-smokers with non-small cell lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement. Cancer 2012;118:729-39. [Crossref] [PubMed]
- Paik JH, Choi CM, Kim H, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer A proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer 2012;76:403-9. [Crossref] [PubMed]
- Kim MH, Shim HS, Kang DR, et al. Clinical and prognostic implications of ALK and ROS1 rearrangements in never-smokers with surgically resected lung adenocarcinoma. Lung Cancer 2014;83:389-95. [Crossref] [PubMed]
- Tantraworasin A, Lertprasertsuke N, Kongkarnka S, et al. Retrospective Study of ALK Rearrangement and Clinicopathological Implications in Completely Resected Non-small Cell Lung Cancer Patients in Northern Thailand: Role of Screening with D5F3 Antibodies. Asian Pac J Cancer Prev 2014;15:3057-63. [Crossref] [PubMed]
- Blackhall FH, Peters S, Bubendorf L, et al. Prevalence and Clinical Outcomes for Patients With ALK-Positive Resected Stage I to III Adenocarcinoma: Results From the European Thoracic Oncology Platform Lungscape Project. J Clin Oncol 2014;32:2780. [Crossref] [PubMed]
- Ohba T, Toyokawa G, Osoegawa A, et al. Mutations of the EGFR, K-ras, EML4-ALK, and BRAF genes in resected pathological stage I lung adenocarcinoma. Surgery Today 2016;46:1091-8. [Crossref] [PubMed]
- Shin SH, Lee H, Jeong BH, et al. Anaplastic lymphoma kinase rearrangement in surgically resected stage IA lung adenocarcinoma. J Thorac Dis 2018;10:3460-7. [Crossref] [PubMed]
- Gao Q, Li P, Jiang X, et al. Worse disease-free, tumor-specific, and overall survival in surgically-resected lung adenocarcinoma patients with ALK rearrangement. Oncotarget 2017;8:86066-81. [Crossref] [PubMed]
- Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique Clinicopathologic Features Characterize ALK-Rearranged Lung Adenocarcinoma in the Western Population. Clin Cancer Res 2009;15:5216-23. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical Features and Outcome of Patients With Non-Small-Cell Lung Cancer Who Harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Sacher AG, Dahlberg SE, Heng J, et al. Association Between Younger Age and Targetable Genomic Alterations and Prognosis in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:313-20. [Crossref] [PubMed]
- Popat S, Gonzalez D, Min T, et al. ALK translocation is associated with ALK immunoreactivity and extensive signet-ring morphology in primary lung adenocarcinoma. Lung Cancer 2012;75:300-5. [Crossref] [PubMed]
- Gainor JF, Varghese AM, Ou SHI, et al. ALK Rearrangements Are Mutually Exclusive with Mutations in EGFR or KRAS: An Analysis of 1,683 Patients with Non-Small Cell Lung Cancer. Clin Cancer Res 2013;19:4273-81. [Crossref] [PubMed]
- Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 1994;263:1281-4. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Woo CG, Seo S, Kim SW, et al. Differential protein stability and clinical responses of EML4-ALK fusion variants to various ALK inhibitors in advanced ALK-rearranged non-small cell lung cancer. Ann Oncol 2017;28:791-7. [PubMed]
- Yoshida T, Oya Y, Tanaka K, et al. Differential Crizotinib Response Duration Among ALK Fusion Variants in ALK-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3383-9. [Crossref] [PubMed]
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203. [Crossref] [PubMed]
- Takeuchi K, Choi YL, Togashi Y, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res 2009;15:3143-9. [Crossref] [PubMed]
- Chiarle R, Voena C, Ambrogio C, et al. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer 2008;8:11-23. [Crossref] [PubMed]
- Soda M, Takada S, Takeuchi K, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A 2008;105:19893-7. [Crossref] [PubMed]
- Yang P, Kulig K, Boland JM, et al. Worse Disease-Free Survival in Never-Smokers with ALK plus Lung Adenocarcinoma. J Thorac Oncol 2012;7:90-7. [Crossref] [PubMed]
- Fukui T, Yatabe Y, Kobayashi Y, et al. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer 2012;77:319-25. [Crossref] [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [Crossref] [PubMed]
- Chaft JE, Dagogo-Jack I, Santini FC, et al. Clinical outcomes of patients with resected, early-stage ALK-positive lung cancer. Lung Cancer 2018;122:67-71. [Crossref] [PubMed]
- McDermott U, Iafrate AJ, Gray NS, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res 2008;68:3389-95. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus Chemotherapy in Advanced ALK-Positive Lung Cancer. New Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. New Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Yang JCH, Ou SHI, De Petris L, et al. Pooled Systemic Efficacy and Safety Data from the Pivotal Phase II Studies (NP28673 and NP28761) of Alectinib in ALK-positive Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:1552-60. [Crossref] [PubMed]
- Camidge DR, Kono SA, Lu XA, et al. Anaplastic Lymphoma Kinase Gene Rearrangements in Non-small Cell Lung Cancer are Associated with Prolonged Progression-Free Survival on Pemetrexed. J Thorac Oncol 2011;6:774-80. [Crossref] [PubMed]
- Possidente L, Landriscina M, Patitucci G, et al. ALK rearrangement in specific subtypes of lung adenocarcinoma: immunophenotypic and morphological features. Med Oncol 2017;34:76. [Crossref] [PubMed]
- Dong YJ, Cai YR, Zhou LJ, et al. Association between the histological subtype of lung adenocarcinoma, EGFR/KRAS mutation status and the ALK rearrangement according to the novel IASLC/ATS/ERS classification. Oncol Lett 2016;11:2552-8. [Crossref] [PubMed]
- Zhao F, Xu M, Lei H, et al. Clinicopathological Characteristics of Patients with Non-Small-Cell Lung Cancer Who Harbor EML4-ALK Fusion Gene: A Meta-Analysis. PLoS One 2015;10:e0117333. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004-12. [Crossref] [PubMed]
- Wu SG, Kuo YW, Chang YL, et al. EML4-ALK Translocation Predicts Better Outcome in Lung Adenocarcinoma Patients with Wild-Type EGFR. J Thorac Oncol 2012;7:98-104. [Crossref] [PubMed]
- von der Thusen JH, Tham YS, Pattenden H, et al. Prognostic Significance of Predominant Histologic Pattern and Nuclear Grade in Resected Adenocarcinoma of the Lung Potential Parameters for a Grading System. J Thorac Oncol 2013;8:37-44. [Crossref] [PubMed]
- Makinen JM, Laitakari K, Johnson S, et al. Nonpredominant lepidic pattern correlates with better outcome in invasive lung adenocarcinoma. Lung Cancer 2015;90:568-74. [Crossref] [PubMed]
- Murakami S, Ito H, Tsubokawa N, et al. Prognostic value of the new IASLC/ATS/ERS classification of clinical stage IA lung adenocarcinoma. Lung Cancer 2015;90:199-204. [Crossref] [PubMed]


