Regionalization of thoracic surgery improves short-term cancer esophagectomy outcomes
Introduction
Esophageal cancer carries a grim prognosis despite advances in treatment made over the last few decades, with a 5-year survival rate for all-comers of only 18.8% (1). Esophagectomy is a key component of care in patients who are candidates for curative treatment, whether as part of multimodal therapy or as primary treatment in early-stage disease, but even after resection, 5-year survival is poor. Esophagectomy is additionally associated with substantial morbidity. Complication rates range from 40% to almost 80% in meta-analyses and database analysis studies, and most individual studies report rates over 50% (2,3). With such high complication rates, and with poor long-term survival placing a higher premium on quality of life, even incremental improvements in postoperative outcomes become important.
A number of studies have found that cancer esophagectomies at higher-volume centers are associated with lower short-term morbidity and mortality than at low-volume centers (4-6). Although the findings are not universal, the evidence for a volume-outcome relationship was sufficient to prompt major policy changes in some countries with nationalized healthcare models, including Great Britain, Canada, the Netherlands, Sweden, and Denmark. Reports on the results of centralization in these systems have largely demonstrated that shifting cases to regional, high-volume hospitals does improve outcomes, and that this phenomenon may be at least partially independent of changes in volume distribution (7-12).
In the United States (U.S.), proactive attempts at regionalization on a large scale have not yet occurred. Efforts have been limited to voluntary programs identifying centers that meet volume thresholds (e.g., Leapfrog Group) or cancer treatment quality standards (e.g., the U.S. National Cancer Institute’s designated Cancer Centers) and, so far, data demonstrating that such movements have changed referral practices or impacted outcomes for patients is unconvincing (13,14). Considering how much American healthcare differs from that of the centralized models abroad, longitudinal evidence reproducing the beneficial effects of regionalization in this setting is needed.
Kaiser Permanente is one of the largest and most successful integrated healthcare networks in the U.S. In its home Northern California region, nearly 9,000 physicians serve 4.3 million members (15), constituting nearly half the region’s insurance market share (16). Kaiser Permanente Northern California (KPNC) has been compared to the British National Health System, and the member pool can be considered reasonably representative of local population demographics (17,18). The KPNC infrastructure enabled the regionalization in 2014 of cardiothoracic surgical services. We examined the impact of regionalizing thoracic surgery on overall care and outcomes for patients undergoing cancer esophagectomy.
Methods
Study design & patient selection
We conducted a retrospective chart review of all adult cancer esophagectomy cases completed within KPNC between 2009 and 2016. Thoracic surgery regionalization was implemented in January 2014 and consolidated cases to 4 of the region’s 21 hospitals, designated as Centers of Excellence (CoE). We compared historical cohorts of patients who underwent surgery before regionalization [2009–2013] to those after regionalization [2014–2016].
Regionalization
The impetus to regionalize was primarily outcomes-motivated and surgeon-driven. The physician group started to track esophagectomy outcomes within KPNC, and the findings were discussed among all surgeons who were performing the operation. Through ongoing dialogue, they developed a plan to improve patient outcomes. A key feature of their proposal to regionalize was that the process would be strictly voluntary. As the only stipulation of regionalization, all surgeons were asked to perform their esophagectomy cases at one of the four CoE. Local surgeons would assist with caring for their patients postoperatively at the remote site. No restrictions were applied to surgeon specialty or esophagectomy method, as there was substantial heterogeneity in techniques (though most were variants of an Ivor-Lewis approach). Furthermore, no contractual penalties were levied against non-compliant surgeons who continued to perform esophagectomies at non-CoE, nor were any incentives granted for compliance or increased volume.
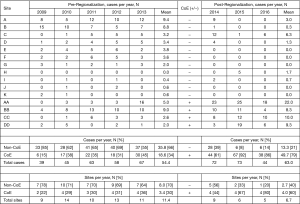
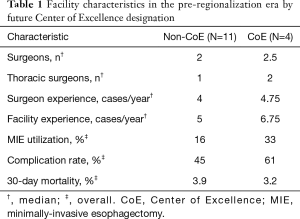
CoE designation was only partially based on established esophagectomy outcomes. Other important considerations included: availability of surgeons who could offer patients a minimally-invasive option; surgeon interest in continuing to perform esophagectomy; local surgeon availability and willingness to assist with postoperative care for esophagectomy cases migrated to their center; operating room (OR) capacity sufficient to absorb additional case-load; adequate nursing and facility resources for postoperative care of esophagectomy patients; and geography within the region. To minimize distance as a barrier to access, sites were selected such that a CoE would be available to patients within 70 miles of their usual primary hospital. Access was further protected by the existing KPNC policy requiring cancer operations to be available to patients within 2 weeks of completion of preoperative workup. Ultimately, decisions on CoE selection were multifactorial and collaborative. The effect of this non-algorithmic, group decision-making process is illustrated by the lack of apparent, quantifiable differences in the pre-regionalization characteristics of future CoE and non-CoE (Table 1).
Full table
Data collection
The cohort was identified through a combination of automated review of coded electronic data and manual researcher review of descriptive data. First, cases were selected from operational databases by diagnosis and procedure codes. These charts were then reviewed by surgeon researchers to verify that the cases met study inclusion criteria and that the esophageal cancer diagnoses were validated against a Cancer Registry maintained by the KPNC Division of Research (DoR), from which neoadjuvant treatment data was also obtained. Patient demographics (age, sex, race/ethnicity) and surgical data including American Society of Anesthesiologists (ASA) classification, surgeon, facility, operating room (OR) time, surgical approach as open or minimally-invasive esophagectomy (OE, MIE), length of stay (LOS), and 30-day readmissions were abstracted electronically by study programmers or by manual chart review. Tumor-node-metastasis (TNM) stage according to American Joint Committee on Cancer Cancer Staging Manual 7th edition guidelines (19) and tumor location (gastro-esophageal junction, lower esophagus, mid esophagus, or upper esophagus) were determined through review of preoperative imaging and endoscopic studies. Carcinoma type/morphology (adenocarcinoma versus squamous cell carcinoma) was collected from the final surgical pathology report. Charts were manually reviewed to determine the incidence of complications within 30 days of surgery: reoperation, anastomotic leak, re-intubation, mechanical ventilation >48 hours, pneumonia, new atrial fibrillation, acute renal failure, and any recurrent laryngeal nerve injury. These complications were selected based on a recent report from the Society of Thoracic Surgeons (STS) database describing factors predictive of morbidity and mortality following esophageal cancer resection (20).
Statistical analysis
Patient, surgeon, and operation characteristics were described in the pre- and post-regionalization periods and compared using Chi-square or Fisher’s exact tests for categorical variables and a Kruskal-Wallis test for age, a non-normally distributed continuous variable. Surgeon and hospital volume were defined as the number of esophagectomies performed each calendar year and assigned to each patient by year of the surgery. Both were analyzed as categorical variables, defined as <5 cases/year versus ≥5 cases/year. Selection of the hospital volume threshold was informed by a median annual case volume for U.S. hospitals performing esophagectomy of only 2 (with 59% of the hospitals performing ≤2) from a 2015 analysis of National Inpatient Survey data (21), taken together with the observed inflection point in decreasing morbidity corresponding to increasing volume at the 5–7 esophagectomies/year quintile in Birkmeyer et al.’s seminal volume-outcome paper (4). We also considered the hospital case volumes observed in our data set such that the selected cutoff would produce a meaningful division among the sites. Less data exists in the literature regarding a surgeon volume threshold reliably associated with differences in outcome and so we utilized both our observed regional surgeon-case volume distribution and prior cutoffs identified in a large meta-analysis (5).
Primary study endpoints were patient LOS (defined as the time from exiting the OR to final hospital discharge order) and any complication. LOS was modeled as a continuous variable and, due to its skewed nature, analysis was restricted to patients with LOS <30 days (96% of the cohort; for this model only). Models for OR time, also as a continuous variable, were stratified by surgical approach. Any complication was modeled as a binary variable using logistic regression. We used hierarchical linear and logistic mixed models to determine change in outcomes pre- to post-regionalization, while controlling for patient-, surgeon-, and hospital-level effects, as well as for clustering within surgeon and facility levels. Mortality rates at 30 and 90 days after surgery were characterized descriptively.
Results
Cohort demographics
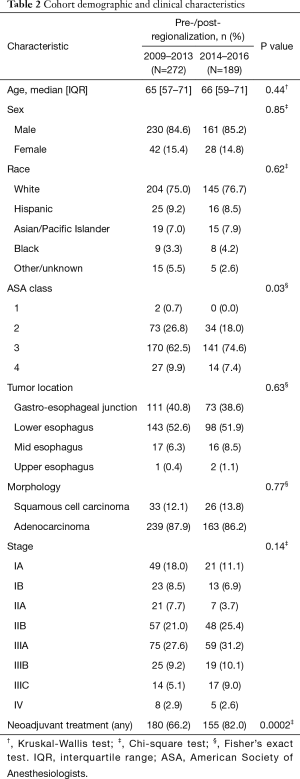
During the study period, 513 patients underwent esophagectomy. One patient was excluded due to age; 36 because they lacked a corresponding cancer diagnosis before surgery; and an additional 15 based on final diagnosis or comorbid pathology detected after surgery. Ultimately, 461 patients/esophagectomies met our criteria. No patients were lost to follow-up. The pre-regionalization (N=272) and post-regionalization (N=189) cohorts did not differ significantly in demographic characteristics (age, gender, race) or cancer epidemiology (tumor location, cancer type) (Table 2). The cohorts did differ with respect to ASA classification, TNM stage, and neoadjuvant treatment. The post-regionalization group comprised more patients in ASA class 3 and less in ASA class 1 or 2 (P=0.03). The post-regionalization group also included patients with more advanced-stage disease, particularly stage II–III (P=0.14). Although the Chi-square test for difference in stage was not statistically significant, the Mantel-Haenszel test for linear trend was (P=0.01). Use of preoperative chemotherapy and/or radiation increased over the study period, such that a higher proportion of post-regionalization patients received neoadjuvant treatment (82.0% versus 66.2%, P=0.0002).
Full table
Regionalization
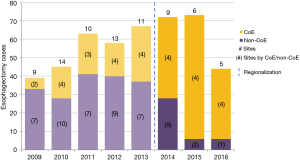
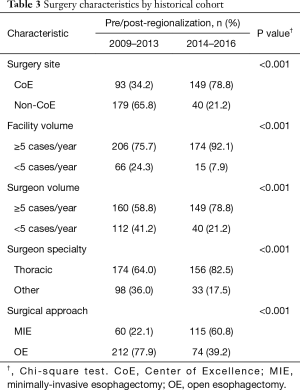
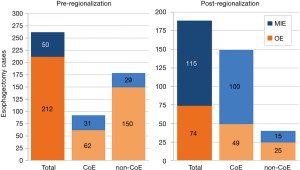
Esophagectomy cases shifted from 24 surgeons across 14 of the region’s 21 hospitals to 18 surgeons across 10 hospitals (total for each era). However, the post-regionalization period was characterized by ongoing evolution in case distribution (Figure 1). By 2016, the last year of our post-regionalization study period, thoracic surgery services had narrowed to 13 surgeons at 5 facilities (Figure 2). Only 6 of 44 esophagectomies that year were performed at non-CoE, by a single surgeon at a single site. The impact of regionalization was evident in the site and surgeon characteristics pre- and post-regionalization (Table 3). Patients undergoing esophagectomy post-regionalization were much more likely to have had their surgery performed at a designated CoE, at a higher-volume hospital, by a higher-volume surgeon, and by a board-certified thoracic surgeon.
Full table
Surgeon, site, and surgery characteristics
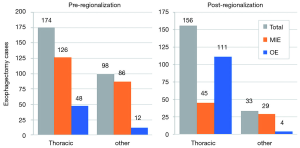
In addition to the changes observed in case distribution, characteristics of the operations changed significantly pre- to post-regionalization (Table 3). Notably, utilization of minimally-invasive approach increased dramatically, with the proportion of patients undergoing MIE rather than OE post-regionalization nearly triple that of pre-regionalization. This effect was even more pronounced in cases done at CoE (Figure 3) or by thoracic surgeons (Figure 4). The thoracic surgeons performed quantitatively and proportionally more MIE than their general surgeon counterparts in both eras. The general surgeons’ low rate of MIE (only 12.2%) did not change at all after regionalization (12.1%)—only 4 MIEs were performed by general surgeons in the post-regionalization era, and only at CoE.
Full table
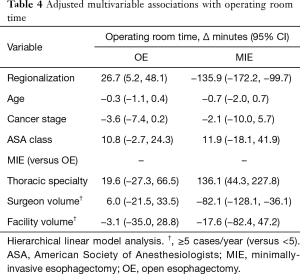
In the fully adjusted model (Table 4), regionalization was associated with a decrease in average MIE OR time of over 2 hours. Decreased MIE OR time was also associated with higher surgeon volume, but not facility volume or surgeon specialty. In fact, thoracic specialty was associated with an increase in MIE OR time. This finding may be somewhat unreliable due to the very low numbers of general surgeon MIE cases (n=16 for the entire study period). Regionalization was associated with an increase in average OE operative time of almost half an hour. Surgeon volume, facility volume, and thoracic specialty were not associated with any difference in OE OR time.
Outcomes
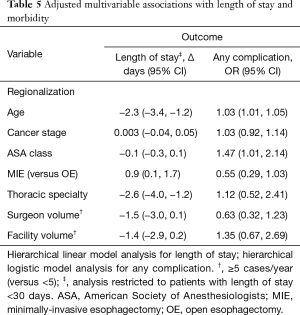
Regionalization was associated with a decrease in LOS of more than 2 days (−2.3 days, 95% CI: −3.4, −1.2 days) in our hierarchical linear model (Table 5), and this was not at the expense of increased readmissions (10.6% from 12.9%, P=0.46). MIE approach was also associated with shorter LOS. There was a borderline association between decreased LOS and surgery performed at a higher-volume facility or by a thoracic surgeon, but not by a higher-volume surgeon.
Full table
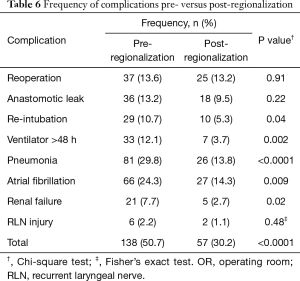
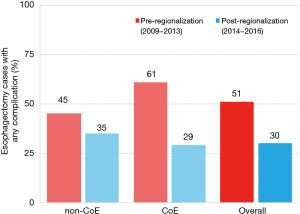
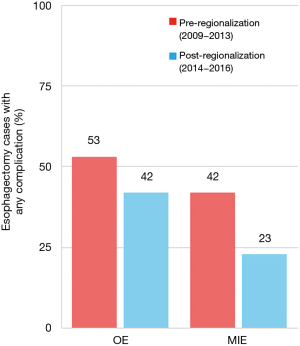
There was a very significant decrease in overall 30-day morbidity pre- to post-regionalization from 50.7% to 30.2% (Table 5). We observed significant reductions in the frequency of many of the individual complications as well (Table 6). The reduction in perioperative morbidity between the study periods was greater at CoE (Figure 5), in both absolute (–19% in CoE versus –11% non-CoE) and relative difference (by 45% in CoE versus by 21% non-CoE). There was no association between decreased complication risk and surgeon volume, facility volume, or surgeon specialty in our adjusted multivariable model. Reduction in perioperative morbidity was greater among MIE cases (Figure 6), and yet the association between MIE approach and decreased 30-day complication was not significant in our model, which controlled for other factors. In fact, regionalization was the only variable significantly associated with decreased complication rate in our fully adjusted model.
Full table
Among specific complications, the greatest absolute risk reduction occurred in pneumonia: a decrease of 16.0%, or >50% reduction in frequency, after regionalization (13.8% from 29.8%, P<0.0001). This was particularly notable because pneumonia is one of the most common complications after esophagectomy, and its occurrence has been shown to negatively affect long-term survival (22,23). To further investigate the impact of regionalization on this clinically important outcome, we performed an additional analysis substituting pneumonia for any complication in our hierarchical logistic model. The adjusted analysis revealed that regionalization was only modestly associated (OR 0.50, 95% CI: 0.24, 1.05), and MIE strongly associated, with decreased risk of pneumonia (OR 0.26, 95% CI: 0.11, 0.60).
Only two factors were associated with an increased complication rate in the fully adjusted model. There was a modest increase with higher ASA and a significant increase with older age.
Because of the marked difference in neoadjuvant treatment rates pre- versus post-regionalization, we re-analyzed the above multivariable models with neoadjuvant therapy included. This did not result in any significant changes to our reported outcomes (<5% difference in OR); therefore, only results from the original models are shown.
Mortality, at both 30 and 90 days, decreased modestly after regionalization: from 3.7% to 2.7% (P=0.54) and 5.9% to 4.8% (P=0.60), respectively. Mortality rates were low in both eras, and the study was not powered to evaluate this difference.
Discussion
Outcomes
Thoracic surgery regionalization resulted in short-term outcomes improvements that were statistically and clinically significant. Our post-regionalization 30-day complication rate (30.2%) and mortality (2.7%) compare favorably to reports in the recent literature from national databases and large, academic, high-volume hospitals of complication rates of 38% to 64% and mortality of 1.7% to 3.8% (2,3,24,25). The achievement by lower-volume, non-academic hospitals within our region of outcomes on par with those from large, university centers suggests that regionalization may be a way for community hospitals to bridge the volume-outcome gap. That none of the volume-related variables (surgeon volume, facility volume, and surgeon specialty) were associated with decreased complication rates after we controlled for other factors emphasizes the impact of regionalization itself.
An unanticipated difference in pre- versus post-regionalization care was the shift toward minimally-invasive surgical approach. The change seems to have been driven by a practice shift among the thoracic surgeons, with a complete reversal of their case mix of 27.5% MIE and 72.5% OE pre-regionalization, to 71.2% MIE and 28.8% OE post-regionalization. Meanwhile, general surgeons performed the same proportion of the total OEs pre- and post-regionalization (39% from 40.4%) but almost none of the MIEs (3.5% from 20%) (Figure 4). These findings provide evidence that regionalization can specifically increase utilization of minimally-invasive techniques, which can be considered a secondary benefit to patients. Numerous studies, including a multi-center randomized, controlled trial, have demonstrated that MIE is associated with decreased LOS and postoperative morbidity, particularly pulmonary complications (2,3,24-27). On the other hand, the improvement in complication rates we observed could have been driven by the relative increase in lower-morbidity MIE cases. However, the association of regionalization and decreased morbidity was independent of MIE in our adjusted multivariable model, and there was no association between surgical approach and overall morbidity (Table 5). Interestingly, the effect was actually more pronounced among MIE cases (Figure 6), with greater absolute and relative reductions in complication rates than in OE cases (MIE versus OE: 19% versus 11%, absolute; 45% versus 21%, relative). The relative contributions of regionalization and surgical approach may vary for individual complications, as MIE did have a stronger association with decreased pneumonia risk than regionalization. It therefore seems likely that alterations in surgical technique played some role in minimizing complications, but that the shift in case mix alone would not fully account for these findings.
Just as the effect of regionalization on surgical approach was enhanced at CoE and blunted at non-CoE (Figure 3), the reduction in morbidity that occurred with regionalization was more pronounced at CoE than at non-CoE. Despite that overall outcomes at future CoE sites were actually worse than non-CoE prior to regionalization, they still achieved a lower complication rate than non-CoE after regionalization (Figure 5). This is especially remarkable given the higher level of comorbid disease and more advanced cancer stage among post-regionalization patients. It may be that the increases in volume and surgeon specialization resulted in greater ability post-regionalization to treat higher-complexity patients due to augmented expertise and experience. As a whole, these findings strongly suggest that environmental factors unique to the CoE were contributing to these changes. This multifactorial mechanism of effect is consistent with prior literature describing the complex influence of hospital ecosystem characteristics on outcomes from specialized oncologic operations (12,28,29).
It is important to recognize that, included among these multifactorial mechanisms, institutional changes occurred proactively and then continued reactively throughout the regionalization process. Safer, more efficient clinical systems were developed to match the shifts in care. In-service teaching sessions were held for nurses in the ORs, in post-anesthesia care units, and on the floors regarding post-esophagectomy care; a booklet on this topic was created specifically for CoE. Surgeons performing esophagectomies were given additional training and support through proctoring by higher-volume surgeons. Many of these interventions would have been less efficacious or impossible without first concentrating care to smaller or more localized groups of providers. The impact of these and other additional measures is difficult to evaluate quantitatively but is highlighted by the association of regionalization with multiple outcomes improvements, independent from surgeon and facility experience.
Regionalization
Our study demonstrates the feasibility of thoracic surgery regionalization within an American integrated healthcare model. To our knowledge, this is the first large-scale regionalization of specialized surgical care outside of a national, socialized healthcare system. We speculate that several aspects of KPNC’s integrated-model infrastructure were important for the successful implementation of regionalization. The uncommon compensation structure in the Kaiser Permanente system may also have been instrumental in the acceptance of regionalization among surgeons. Kaiser Permanente physicians, including surgeons, are not reimbursed through a fee-for-service model, which minimizes potential financial disincentives. This may also have fostered cooperation among surgeons by minimizing competition between individuals for cases. Additional systems-based aspects of the KPNC network that supported regionalization include: a well-established and unified EMR, pre-existing institutional mechanisms for transfer of care within the network, and capabilities for secure communication among providers and patients.
This first-in-U.S. proactive, multi-center regionalization of specialized surgical care produced changes that directly and indirectly improved patient outcomes after esophagectomy. Our findings are similar to those from existing reports of outcome improvements following esophagectomy regionalization in international single-payer models (7,10). Some of the reported reductions in short-term mortality were more dramatic than ours, but most of those studies had also reported higher pre-regionalization mortality rates of closer to 10% (7,9,12). Overall, our results are consistent with those in the current literature and provide evidence that regionalization in the U.S. can produce outcomes gains comparable to those seen after Canadian and European centralization. Our model is readily generalizable to the other regions of Kaiser Permanente, which encompass another 7.9 million members (17). Although integrated, managed-care organizations on the scale of Kaiser Permanente are the exception rather than the rule within the heterogeneous, private-payer-dominated U.S. market, lessons from our experience should still be helpful in designing strategies for regionalization appropriate to other practice environments.
Limitations
One major limitation of our study design was the recency of our post-regionalization cohort, which precluded comparison of long-term outcomes. This is of particular concern because the data from centralization in nationalized healthcare systems has been less robust with respect to long-term mortality benefits; only some have showed persistence of mortality benefits at 1–3 years (12,30,31). Beyond that, only the Danish and Swedish have reported evidence of sustained effects on survival at 5 years, and the data was mixed (5,32). Long-term follow-up is needed to assess the oncologic quality of these operations, as well as to ascertain if the early benefits in overall mortality persist over time.
There are other notable limitations to this study. Its retrospective, observational design makes the results susceptible to confounding by a number of variables. In particular, we cannot exclude the influence of ongoing medical advancement, such as increasing surgeon and facility experience over time, evolution in clinical practice, and developments in adjuvant therapy. The difference in neoadjuvant therapy between eras is an example of this effect; the trend in our data parallels that in large, national U.S. databases (33) and reflects shifts in the standard of care within the field (34). A large, multi-center, randomized controlled trial would be needed to exclude these confounding effects.
Finally, concern has been raised about the potential for regionalization to create or exacerbate healthcare disparities, particularly in terms of access to care. Fortunately, the multidisciplinary, patient-centered orientation of the Kaiser Permanente system seems to be protective against this effect. Tools for secure patient-doctor and provider communication are integrated in the EMR, and the culture is such that physicians who share a patient will work collaboratively. In fact, the post-regionalization increases in the average annual case load, patient complexity in terms of comorbid illness and tumor stage, and performance of surgery by specialists suggests that our patients actually had increased access to specialty surgeons and possibly increased access to surgical treatment overall.
Conclusions
Regionalization of thoracic surgery care in our healthcare network resulted in cancer esophagectomies being performed by higher-volume, cardiothoracic-specialized surgeons at higher-volume facilities, with a concomitant improvement in short-term outcomes. Patients undergoing esophagectomy, especially MIE, post-regionalization experienced dramatically reduced LOS and 30-day complication rates. In our adjusted multivariable models, regionalization was the only factor independently associated with both outcomes. Our findings suggest that regionalization resulted in changes that cannot be accounted for by volume alone. This study further demonstrates that, in a large American integrated healthcare system, regionalization of complex cancer surgery can improve short-term patient outcomes.
Acknowledgments
This research was supported by a Kaiser Permanente Division of Research Community Benefit grant.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Kaiser Permanente Institutional Review Board (IRB), under protocol CN 16-2724.
References
- Cancer Stat Facts: Esophageal Cancer [Internet]. National Cancer Institute: Surveillance, Epidemiology, and End Results Program [cited 2017 Oct 15]. Available online: https://seer.cancer.gov/statfacts/html/esoph.html
- Gemmill EH, Mcculloch P. Systematic review of minimally invasive resection for gastro-oesophageal cancer. Brit J Surg 2007;94:1461-7. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. New Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Brusselaers N, Mattsson F, Lagergren J. Hospital and surgeon volume in relation to long-term survival after oesophagectomy: systematic review and meta-analysis. Gut 2014;63:1393-400. [Crossref] [PubMed]
- Markar S, Gronnier C, Duhamel A, et al. Pattern of postoperative mortality after esophageal cancer cesection according to center volume: results from a large European multicenter study. Ann Surg Oncol 2015;22:2615-23. [Crossref] [PubMed]
- Bachmann MO, Alderson D, Edwards D, et al. Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Brit J Surg 2002;89:914-22. [Crossref] [PubMed]
- Rouvelas I, Zeng W, Lindblad M, et al. Survival after surgery for oesophageal cancer: a population-based study. Lancet Oncol 2005;6:864-70. [Crossref] [PubMed]
- Wouters MWJM, Karim-Kos HE, Cessie SL, et al. Centralization of esophageal cancer surgery: Does it improve clinical outcome? Ann Surg Oncol 2009;16:1789-98. [Crossref] [PubMed]
- Finley CJ, Jacks L, Keshavjee S, et al. The effect of regionalization on outcome in esophagectomy: a Canadian national study. Ann Thorac Surg 2011;92:485-90. [Crossref] [PubMed]
- Kjaer DW, Larsson H, Svendsen LB, et al. Changes in treatment and outcome of oesophageal cancer in Denmark between 2004 and 2013. Brit J Surg 2017;104:1338-45. [Crossref] [PubMed]
- Varagunam M, Hardwick R, Riley S, et al. Changes in volume, clinical practice and outcome after reorganisation of oesophago-gastric cancer care in England: A longitudinal observational study. Eur J Surg Oncol 2018;44:524-31. [Crossref] [PubMed]
- Varghese TK, Wood DE, Farjah F, et al. Variation in esophagectomy outcomes in hospitals meeting Leapfrog volume outcome standards. Ann Thorac Surg 2011;91:1003-9. [Crossref] [PubMed]
- Birkmeyer NJO, Goodney PP, Stukel TA, et al. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer 2005;103:435-41. [Crossref] [PubMed]
- Fast Facts About Kaiser Permanente [Internet]. Kaiser Permanente [cited 2018 Mar]. Available online: https://share.kaiserpermanente.org/article/fast-facts-about-kaiser-permanente/
- Market Share and Enrollment of Largest Three Insurers- Individual Market [Internet]. Henry J. Kaiser Foundation, State Health Facts [cited 2018 Feb 23]. Available online: https://www.kff.org/private-insurance/state-indicator/market-share-and-enrollment-of-largest-three-insurers-individual-market/?currentTimeframe=0
- Feachem RGA, Sekhri NK, White KL. Getting more for their dollar: a comparison of the NHS with California's Kaiser Permanente. BMJ 2002;324:135-41. [Crossref] [PubMed]
- McCarthy D, Mueller K, Wrenn J. Kaiser Permanente: bridging the quality divide with integrated practice, group accountability, and health information technology. Commonwealth Fund 2009;17(1278).
- Rice TW, Kelsen D, Blackstone EH, et al. Esophagus and esophagogastric junction. In: Edge SB, Byrd DR, Compton CC, et al. editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer-Verlag, 2009:103-15.
- Raymond DP, Seder CW, Wright CD, et al. Predictors of major morbidity or mortality after resection for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. Ann Thorac Surg 2016;102:207-14. [Crossref] [PubMed]
- Munasinghe A, Markar SR, Mamidanna R, et al. Is it time to centralize high-risk cancer care in the United States? Comparison of outcomes of esophagectomy between England and the United States. Ann Surg 2015;262:79-85. [Crossref] [PubMed]
- Booka E, Takeuchi H, Nishi T, et al. The Impact of Postoperative Complications on Survivals After Esophagectomy for Esophageal Cancer. Medicine 2015;94:e1369. [Crossref] [PubMed]
- Kataoka K, Takeuchi H, Mizusawa J, et al. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer. Ann Surg 2017;265:1152-7. [Crossref] [PubMed]
- Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [Crossref] [PubMed]
- Rodham P, Batty JA, Mcelnay PJ, et al. Does minimally invasive oesophagectomy provide a benefit in hospital length of stay when compared with open oesophagectomy? Interact Cardiovasc Thorac Surg 2016;22:360-7. [Crossref] [PubMed]
- Biere SS, Henegouwen MIVB, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Tapias LF, Mathisen DJ, Wright CD, et al. Outcomes with open and minimally invasive Ivor Lewis esophagectomy after neoadjuvant therapy. Ann Thorac Surg 2016;101:1097-103. [Crossref] [PubMed]
- Kothari AN, Blanco BA, Brownlee SA, et al. Characterizing the role of a high-volume cancer resection ecosystem on low-volume, high-quality surgical care. Surgery 2016;160:839-49. [Crossref] [PubMed]
- Funk LM, Gawande AA, Semel ME, et al. Esophagectomy outcomes at low-volume hospitals: the association between systems characteristics and mortality. Ann Surg 2011;253:912-7. [Crossref] [PubMed]
- Dikken JL, Dassen AE, Lemmens VE, et al. Effect of hospital volume on postoperative mortality and survival after oesophageal and gastric cancer surgery in the Netherlands between 1989 and 2009. Eur J Cancer 2012;48:1004-13. [Crossref] [PubMed]
- Boddy AP, Williamson JM, Vipond MN. The effect of centralisation on the outcomes of oesophagogastric surgery - A fifteen year audit. Int J Surg 2012;10:360-3. [Crossref] [PubMed]
- Storm HH, Gislum M, Kejs AM, et al. Survival of Danish cancer patients 1995-2006. Ugeskr Laeger 2010;172:2213-7. [PubMed]
- Valsangkar N, Salfity HV, Timsina L, et al. Operative time in esophagectomy: Does it affect outcomes? Surgery 2018;164:866-71. [Crossref] [PubMed]
- van Hagen P, Hulshof MCCM, Lanschot JJBV, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. New Engl J Med 2012;366:2074-84. [Crossref] [PubMed]