MicroRNA-374b inhibits the tumor growth and promotes apoptosis in non-small cell lung cancer tissue through the p38/ERK signaling pathway by targeting JAM-2
Introduction
Lung cancer is considered as the most lethal and prevalent cancer worldwide, clinically characterized by high malignancy, high mortality, and low 5-year survival rates (1). The number of lung cancer-related mortalities is more than 1.4 million annually, which is considered the primary form of cancer-related mortality worldwide (2). Non-small cell lung cancer (NSCLC) is closely associated with distant metastasis and is difficult to treat. Currently, surgical treatment, radiotherapy, and chemotherapy are widely used to treat NSCLC. However, the treatment methods for NSCLC have low efficiency and are unable to control tumor progression. Thus, basic studies on NSCLC are critical for its accurate prognosis, early detection, and effective treatment (3). As such, it is vital to understand the mechanism underlying the development of NSCLC and accordingly develop an effective and desirable treatment method.
MicroRNAs (miRNAs) are endogenous single-stranded small noncoding RNAs comprising 18–25 nucleotides (4). They were first reported in Caenorhabditis elegans in 1993 (5). miRNAs bind to the 3'-untranslated regions (UTRs) of multiple target mRNAs and regulate their expression (and concomitant protein expression) by mediating their degradation (6). miRNAs are important regulators of all biological processes, including cell proliferation, differentiation, growth, apoptosis, and senescence, metabolism, infection, and tumor invasion (7). However, miRNAs also participate in the occurrence and progress of various types of diseases, such as osteoporosis, myocardial infarction, diabetes mellitus, and Alzheimer’s disease (8). Further, they are associated with cancer; some miRNAs are tumor suppressors, whereas others are oncogenic, such as miRNA-215 inhibits proliferation, migration, and invasion in NSCLC cells by inhibiting matrix metalloproteinase (MMP)-16, thereby serving as a potential therapeutic target for NSCLC in humans (9). miRNA-155-5p promotes autophagy in cervical cancer cells by targeting PDK1 and further inhibits the tumor progression in cervical cancers. Further, miRNA-216b increases growth, migration, and invasion, and serves as a tumor suppressor in pancreatic ductal adenocarcinoma (10). In addition, miRNA-1269 promotes cell survival and accelerates the proliferative ability of lung cancer cells by inhibiting its target gene TP53 (11).
Furthermore, miRNA expression levels are closely associated with cancers and can be considered markers of certain tumors, e.g., upregulation of miRNA-452-5p can be considered a screening biomarker for lung squamous cell carcinoma, thereby potentially playing a vital role in the development of lung squamous cell carcinoma (12).
As reported previously, miRNA-374b plays a vital role in cancers, including cervical cancer, liver cancer, colon cancer, and colorectal cancer (13,14). Therein, miRNA-374b suppresses tumorigenesis. However, no studies have investigated the effect of miRNA-374b on NSCLC cells. Therefore, the present study aimed to investigate whether miRNA-374b participates in the growth and development of NSCLC and its underlying mechanism and whether it may be considered a therapeutic target for NSCLC.
Methods
Patients and samples
In this study, we collected blood samples and tissue samples from 48 healthy volunteers [n=48, 24 women, 24 men; mean ± standard deviation (SD) age, 42±15 years] and 48 NSCLC patients (n=48, 24 women, 24 men; mean ± SD age, 40±15 years). From January 2016 to May 2017, NSCLC patients were admitted in the Departments of Oncology Division, and Respiratory Medicine of our hospital and the samples from these patients constituted the disease group. Moreover, healthy volunteers without lung cancer and other respiratory diseases constituted the control group. All participants in the disease group were confirmed to have NSCLC via pathological examination and diagnosed by at least two physicians. All participants receiving chemo- or radiotherapy before surgery were excluded. This study was approved by the Ethics Committee of our hospital and written informed consent was provided by all participants and their family numbers. The characteristics of the NSCLC patients are shown in Table 1.
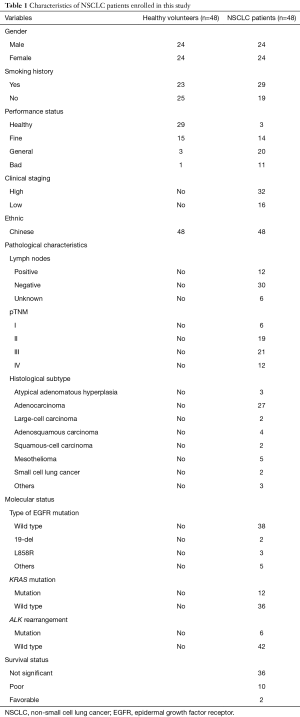
Full table
In accordance with the approved protocol, we collected the blood samples from NSCLC patients and healthy volunteers in the morning. Furthermore, tumor tissues and adjacent non-tumor tissues were harvested from patients who underwent surgery at our hospital. These samples were soaked in RNA later solution (Vazyme, Nanjing, China) and stored at –80 °C immediately and until use.
Cell lines and culture
The human NSCLC cell line A549 (American Type Culture Collection, Manassas, VA, USA) was cultured in Dulbecco’s modified Eagle’s medium (Hyclone, Logan City, Utah, USA) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific, Waltham, Massachusetts, USA) and an antibiotic cocktail comprising 100 U/mL penicillin (Sigma, Saint Louis, California, USA) and 100 µg/mL streptomycin (Sigma Saint Louis, California, USA), in a humidified incubator (Thermo Fisher Scientific).
RNA isolation, cDNA synthesis, and quantitative reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted using TRIzol reagents (Thermo Fisher Scientific) in accordance with the manufacturer’s protocol. The concentration and quality control of total RNAs were determined using a NanoDrop-1000 spectrometer (NanoDrop, Thermo Fisher Scientific, Waltham, Massachusetts, USA). cDNA first-strand synthesis was carried out using a reverse transcription kit (Applied Biosystems, USA) by a reverse transcription machine (Applied Biosystems, Foster City, California, USA). After that, qRT-PCR was performed at 94 °C for 5 min and 42 °C for 30 min, using ChamQTM Universal SYBR® qPCR Master Mix (Vazyme, China). The cycling conditions were as follows: 95 °C for 10 min, followed by 40 cycles at 95 °C for 1 min, 53 °C for 1 min, and 72 °C for 1 min, and 72 °C for 5 min. The primer sequences used in these experiments were as follows: miRNA-374b: 5'-AUAUAAUACAACCUGCUAAGUG-3'; U6: F: 5'-GCTTCGGCAGCACATATACT-3', R: 5'-TTCACGAATTTGCGTGTCAT-3'; GAPDH: F: 5'-TCCACTGGCGTCTTCACC-3', R: 5'-GGCAGAGATGATGACCCTTTT-3'. miRNA-374b expression level was normalized to that of U6. GAPDH was considered the internal control for target genes. We quantified the relative gene expression levels of target genes, using the 2–ΔΔCt method. Each experiment was repeated independently in triplicate.
miRNA transfection
For transfection, miRNA-374b mimics, miRNA-374b anti-miRNA oligonucleotide (AMO), and their corresponding controls were synthesized and provided by RiboBio company (Guangzhou, China). The sequences are as follows: miR-374b mimic, 5'-AUAUAAUACAACCUGCUAAGUG-3'; NC, 5'-UUCUCCGAACGUGUCACGUTT-3'; miR-374b inhibitor, 5'-CACUUAGCAGGUUGUAUUAUAU-3'. miRNA-374b mimics, miRNA-374b anti-miRNA oligonucleotide (AMO), and their corresponding control were transfected into the cells at 50–70% confluency, using transfection reagent X-treme (Vazyme). A549 cells were pretreated with 50 nM of miRNA-374b mimic and 100 nM of miRNA-374b AMO for 24 h for further analysis. The transfection efficiency of miRNA-374b was determined via qRT-PCR. Overexpression was defined as a ≥1.50-fold change; knockdown as ≤0.60-fold change.
MTT assay
Cell proliferation was examined via an MTT assay. Briefly, the NSCLC cells were plated in a 96-well plate and maintained in the culture medium. When the cells were approximately 80% confluent, 20 µL of MTT solution (5 µg/mL, Biasharp, China) was added. The cells were then incubated in MTT dye for 4 h in a 37 °C humidified incubator. Thereafter, we added 100–150 dimethyl sulfoxide (DMSO, Thermo Fisher Scientific) to each well and incubated the plate for 15 min. The absorbance values were detected using a microplate reader (TECAN, Salzburg, Switzerland) at 470 nm. Proliferative ability was determined based on the optical density values. Measurements were obtained for at least three wells, and the experiment was repeated three or more times, independently.
Colony formation assay
NSCLC cells treated with miRNA-374b were trypsinized and replated in 6-well plates. The cells were cultured for 7–10 d and the medium was replenished every 3 d. Subsequently, the cells in a 6-well plate were washed with phosphate-buffered saline (PBS; Beyotime, Shanghai, China) and fixed in 1 mL 4% paraformaldehyde (PFA; Beyotime) for 30 min at 25 °C. The number of colonies in each well was counted and evaluated via 0.5% Crystal Violet staining (Biosharp, Suzhou, Jiangsu, China) for 15 min, using a microscope (Olympus, Tokyo, Japan).
In vivo tumorigenesis assay
Animal experiments conformed to the bioethics guidelines of the laboratory animal ethics committee of our hospital. Forty 6-week-old female immunodeficient nude mice were obtained and fed in accordance with the regulations and internal biosafety and bioethics guidelines of our hospital. After allowing them to acclimatize for one week, the mice were subcutaneously inoculated with A549 cells. On the 14th day, the tumors measured approximately 100 mm3, and miRNA-374b mimics, miRNA-374b AMO and their corresponding controls were injected into the tumors for 4 weeks. After 1 month, the mice were euthanized, and tumor volume was assessed and analyzed.
Terminal deoxynucleotidyl transferase-dUTP nick-end labeling (TUNEL) staining
A549 cells were transfected with miRNA-374b mimics, miRNA-374b AMO, and their corresponding controls for 24 h. Next, the cells were harvested and gently rinsed with PBS thrice. The A549 cells were then fixed in 4% PFA for 30 min, and TUNEL staining was performed. The cells were treated with TUNEL (Roche, Switzerland) by the manufacturer’s protocol. Finally, the apoptotic cells were detected and analyzed using a microscope (Nikon, Tokyo, Japan). The apoptotic cells showed green color while the nuclei stained, blue.
Western blot analysis
The total proteins were isolated from transfected A549 cells, using radioimmunoprecipitation assay solution (RIPA, Beyotime, China). Protein expression levels were assessed via western blotting in accordance with previously reported methods (15,16). The primary anti-JAM-1 (1:500) and anti-GAPDH (1:500) antibodies and their corresponding secondary antibody (1:500) were obtained from Santa Cruz Biotechnology (Shanghai, China). GAPDH was considered the loading control. Bands were detected using the Odyssey machine and analyzed using Image Studio Software (LI-COR Bioscience, Lincoln, Nebraska, USA).
Statistical analysis
All data were analyzed from three or more independent experiments. Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, California, USA). Differences in study variables were considered statistically significant when *P<0.05, **P<0.01 and ***P<0.001. Statistical differences were determined by Student’s t-test between two groups.
Results
miRNA-374b expression in NSCLC patients
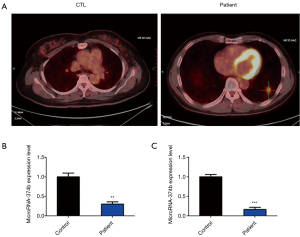
Previous studies have reported the role of miRNA-374b in cancers; however, it is unclear whether miRNA-374b is associated with NSCLC. To evaluate this association, we collected blood samples from healthy volunteers and NSCLC patients in our hospital and then investigated whether miRNA-374b is dysregulated in the blood samples from these two groups. First, the diagnosis of NSCLC was confirmed via positron emission tomography with computed tomography (PET-CT). Compared with the PET-CT results of healthy controls, those for initial staging of NSCLC patients revealed the presence of irregular nodules of the base segment of the lower lobe of the left lung, measuring approximately 1.4×2.6 cm2, accompanied by blurred edges and considered as lung cancer (Figure 1A). qRT-PCR analysis revealed that the miRNA-374b was significantly downregulated in the blood samples of NSCLC patients (**P<0.01) (Figure 1B). Furthermore, for baseline control, miRNA-374b expression level was analyzed in the corresponding normal tissue from the same patient. qRT-PCR results indicated that miRNA-374b was significantly downregulated in the NSCLC tissues as compared to that in the normal adjacent non-tumor tissues from NSCLC patients, as well (***P<0.001) (Figure 1C).
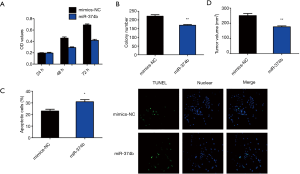
Role of miRNA-374b mimics in NSCLC cells
To analyze whether miRNA-374b affects NSCLC cells, we transfected A549 cells with miRNA-374b mimic and its negative control for 24 h. MTT assay revealed that miRNA-374b significantly suppressed the viability of NSCLC cells compared to that of NC (***P<0.001) (Figure 2A). We then assessed colony formation to determine the role of miRNA-374b in the long-term proliferation of NSCLC cells. miRNA-374b prominently suppressed clonogenic growth and even the proliferative ability of NSCLC cells (Figure 2B). Furthermore, miRNA-374b drastically augmented the number of apoptotic cells compared with that in the NC, as evident from TUNEL staining, (*P<0.05) (Figure 2C). Upon in vivo evaluation, as shown in Figure 2D, after 4 weeks of injection, tumor volume in the miRNA-374b group was significantly less than that in the control group (**P<0.01). The tumorigenesis assay revealed that miRNA-374b overexpression markedly inhibited tumor formation in NSCLC in vivo (Figure 2D). Per the aforementioned results, we confirmed that miRNA-374b treatment effectively decreases cell viability, proliferation, and tumor formation and promotes apoptosis in NSCLC, thereby potentially serving as a tumor suppressor in NSCLC.
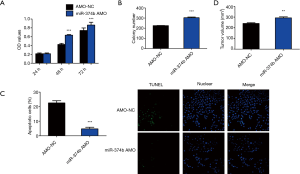
Effect of miRNA-374b AMO on NSCLC cells
To further investigate whether miRNA-374b regulates cell viability, proliferation, apoptosis, and tumorigenesis in NSCLC, we investigated the effect of miRNA-374b AMO on NSCLC cells. miRNA-374b AMO increased cell viability, confirmed via the MTT assay (Figure 3A). To further investigate whether knockdown of miRNA-374b increased proliferation of NSCLC cells, we performed a colony formation assay. miRNA-374b enhanced the growth and proliferation of NSCLC cells (Figure 3B). Moreover, the group pretreated with miRNA-374b AMO contained fewer TUNEL-positive cells than the control group, suggesting that miRNA-374b significantly suppressed apoptosis in NSCLC cells (***P<0.001) (Figure 3C). Mice injected with miRNA-374b AMO had significantly larger tumors than the control mice (**P<0.01) (Figure 3D). Thus, miRNA-374b promotes tumor cell proliferation and tumorigenesis in NSCLC; however, it reduces apoptosis in NSCLC.
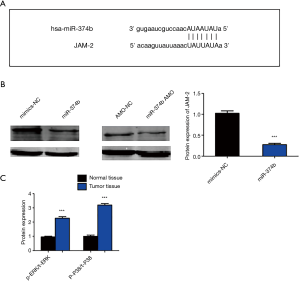
miRNA-374b inhibits NSCLC in a JAM-2-dependent manner
Putative target genes of miRNA-374b were predicted using TargetScan website online (http://www.targetscan.org/vert_72/). Among the numerous targets, junctional adhesion molecule-2 (JAM-2) is associated with cancers and is an oncoprotein in gastric adenocarcinoma. Therefore, we selected JAM2 as the candidate target mRNA for miRNA-374b for further analysis. The binding sites between miRNA-374b and JAM-2 are shown in Figure 4A. Western blotting revealed that miRNA-374b significantly downregulated JAM-2 (Figure 4B) (***P<0.001). Thus, the present results suggest that miRNA-374b regulates cell viability, proliferation, tumor formation, and apoptosis by targeting JAM2 mRNA. Western blotting revealed that p-P38/t-P38 and p-ERK/t-ERK were significantly upregulated in the tumor tissues compared to that in normal the tissues in the same NSCLC patients (Figure 4C). These results indicate that miRNA-374b inhibits tumor growth and promotes apoptosis through the p38/ERK signaling pathway by targeting JAM-2 in NSCLC.
Discussion
NSCLC is the primary cause of death among all types of lung cancer, resulting in heavy economic burden and great pain to patients and their families (17,18). At present, adjuvant platinum-based combinatorial chemotherapeutic methods are widely used in clinical practice (19). However, the present treatment methods are unable to effectively improve overall survival and efficiently control the progression of lung cancer (20). Therefore, understanding the molecular mechanism underlying the occurrence and progression of NSCLC is important to treat such patients (21). Moreover, it is important to determine crucial biomarkers for NSCLC to enhance its early diagnosis and treatment.
The present study investigated the role of miRNA-374b in cell viability, proliferation, apoptosis, and tumor formation and its underlying molecular mechanisms in NSCLC cells. miRNA-374b reportedly participates in the occurrence, progression, and proliferation of tumors (22). Moreover, miRNA-374b plays an important role in myoblast differentiation, immune response, and nephropathy regulation. However, it has been unclear whether miRNA-374b is dysregulated in the blood and tissue samples of NSCLC patients and whether it regulates proliferation and apoptosis of NSCLC cells.
Our results show that miRNA-374b was downregulated in the blood samples of NSCLC patients compared to that in healthy controls. Furthermore, the qRT-PCR analysis revealed that miRNA-374b was significantly downregulated in tumor tissues in comparison with that in normal tissues in the same NSCLC patients. MTT assay revealed that miRNA-374b suppressed the viability of NSCLC cells, and colony formation assay revealed that miRNA-374b inhibited the growth and proliferation of NSCLC cells. In addition, miRNA-374b decreased tumor formation and promoted apoptosis in NSCLC cells. Moreover, miRNA-374b exhibited tumor inhibition in NSCLC.
Furthermore, we determined the targets of miRNA-374b, using TargetScan. JAM-2 was found to be a candidate target of miRNA-374b, containing the conserved sites in the 3'-UTR. The JAM family is reportedly associated with the formation of tight junctions and certain cancers (23). JAM-A, JAM-B (also called JAM-2), JAM-C, and JAM-L belong to the JAM protein family. Western blotting revealed that miRNA-374b downregulated JAM2 mRNA. Thus, miRNA-374b regulates cell viability, proliferation, and apoptosis by directly targeting JAM-2.
The major limitation of this study is the small cohort size; further studies with a larger patient cohort and more patient samples are required to determine whether miRNA-374b levels in the blood is an effective predictive biomarker for NSCLC. Furthermore, additional analyses are required to determine the role of miRNA-374b in NSCLC. In a future study, we intend to collect more human samples to determine whether miRNA-374b can serve as a clinical biomarker for NSCLC.
Conclusions
In summary, the present results show that miRNA-374b is significantly downregulated in the blood and tissues of NSCLC patients. These results provide a novel index for the prediction and diagnosis of NSCLC in clinical practice. We showed that miRNA-374b inhibits cell viability, proliferation, and tumorigenesis in NSCLC and promotes apoptosis in these cells. Furthermore, we demonstrated that upregulation of miRNA-374b inhibited NSCLC in a JAM-2-dependent manner through the p38/ERK signaling pathway. Thus, miRNA-374b is a critical and potential therapeutic target for NSCLC. Our study hence indicates a putative useful biomarker and an experimental basis for the clinical treatment of NSCLC.
Acknowledgements
Funding: Sincere thanks are given to Research Institute of Medicine and Pharmacy, Qiqihar Medical University. This study was supported by the Natural Science Foundation of Hainan, China (2018CXTD347) and National Natural Science Foundation of China (81671771).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of our hospital and written informed consent was provided by all participants and their family numbers.
References
- Hao H, Zhou Z, Li S, et al. Shell feature: a new radiomics descriptor for predicting distant failure after radiotherapy in non-small cell lung cancer and cervix cancer. Phys Med Biol 2018;63. [Crossref] [PubMed]
- Zhou C. Lung cancer molecular epidemiology in China: recent trends. Transl Lung Cancer Res 2014;3:270-9. [PubMed]
- Moeller B, Balagamwala EH, Chen A, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol 2018;8:245-50. [Crossref] [PubMed]
- Liu HJ, Liu B. Inhibition of MicroRNA-23 Contributes to the Isoflurane-Mediated Cardioprotection Against Oxidative Stress. Cardiovasc Toxicol 2018;18:450-8. [Crossref] [PubMed]
- Nadeem A, Ashraf MR, Javed M, Hussain T, Tariq MS, Babar ME. Review - MicroRNAs: A new paradigm towards mechanistic insight of diseases. Pak J Pharm Sci 2018;31:2017-26. [PubMed]
- Wang W, Li DB, Li RY, Zhou X, Yu DJ, Lan XY, Li JP, Liu JL. Diagnosis of Hyperacute and Acute Ischaemic Stroke: The Potential Utility of Exosomal MicroRNA-21-5p and MicroRNA-30a-5p. Cerebrovasc Dis 2018;45:204-12. [Crossref] [PubMed]
- Zhong Y, Du Y, Yang X, et al. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol Cancer 2018;17:79. [Crossref] [PubMed]
- Liu M, Chen Y, Huang B, et al. Tumor-suppressing effects of microRNA-612 in bladder cancer cells by targeting malic enzyme 1 expression. Int J Oncol 2018;52:1923-33. [PubMed]
- Yao Y, Shen H, Zhou Y, et al. MicroRNA-215 suppresses the proliferation, migration and invasion of non-small cell lung carcinoma cells through the downregulation of matrix metalloproteinase-16 expression. Exp Ther Med 2018;15:3239-46. [PubMed]
- Liu YA, Zhang Y, Zheng Z, et al. MicroRNA-216b reduces growth, migration and invasion of pancreatic ductal adenocarcinoma cells by directly targeting ρ-associated coiled-coil containing protein kinase 1. Oncol Lett 2018;15:6745-51. [PubMed]
- Bao M, Song Y, Xia J, et al. miR-1269 promotes cell survival and proliferation by targeting tp53 and caspase-9 in lung cancer. Onco Targets Ther 2018;11:1721-32. [Crossref] [PubMed]
- Gan XN, Gan TQ, He RQ, et al. Clinical significance of high expression of miR-452-5p in lung squamous cell carcinoma. Oncol Lett 2018;15:6418-30. [PubMed]
- Li GC, Cao XY, Li YN, et al. MicroRNA-374b inhibits cervical cancer cell proliferation and induces apoptosis through the p38/ERK signaling pathway by binding to JAM-2. J Cell Physiol 2018;233:7379-90. [Crossref] [PubMed]
- Huang F, Wang B, Zeng J, et al. MicroRNA-374b inhibits liver cancer progression via down regulating programmed cell death-1 expression on cytokine-induced killer cells. Oncol Lett 2018;15:4797-804. [PubMed]
- Zhou P, Hu J, Wang X, et al. Epidermal growth factor receptor expression affects proliferation and apoptosis in non-small cell lung cancer cells via the extracellular signal-regulated kinase/microRNA 200a signaling pathway. Oncol Lett 2018;15:5201-7. [PubMed]
- Chen W, Zhu H, Yin L, et al. lncRNA-PVT1 Facilitates Invasion Through Upregulation of MMP9 in Nonsmall Cell Lung Cancer Cell. DNA Cell Biol 2017;36:787-93. [Crossref] [PubMed]
- Aruna, Li LM. Overexpression of golgi membrane protein 1 promotes non-small-cell carcinoma aggressiveness by regulating the matrix metallopeptidase 13. Am J Cancer Res 2018;8:551-65. [PubMed]
- Attili I, Karachaliou N, Bonanno L, et al. STAT3 as a potential immunotherapy biomarker in oncogene-addicted non-small cell lung cancer. Ther Adv Med Oncol. 2018;10. [Crossref] [PubMed]
- Hutchings D, Maleki Z, Rodriguez EF. Pulmonary Non-Small Cell Carcinoma With Morphologic Features of Adenocarcinoma or “Non-Small Cell Carcinoma Favor Adenocarcinoma” in Cytologic Specimens Share Similar Clinical and Molecular Genetic Characteristics. Am J Clin Pathol 2018;149:514-21. [Crossref] [PubMed]
- Wang XH, Cui YX, Wang ZM, et al. Down-regulation of FOXR2 inhibits non-small cell lung cancer cell proliferation and invasion through the Wnt/β-catenin signaling pathway. Biochem Biophys Res Commun 2018;500:229-35. [Crossref] [PubMed]
- You J, Cheng J, Yu B, et al. Baicalin, a Chinese Herbal Medicine, Inhibits the Proliferation and Migration of Human Non-Small Cell Lung Carcinoma (NSCLC) Cells, A549 and H1299, by Activating the SIRT1/AMPK Signaling Pathway. Med Sci Monit 2018;24:2126-33. [Crossref] [PubMed]
- Ma Z, Sun X, Xu D, et al. MicroRNA, miR-374b, directly targets Myf6 and negatively regulates C2C12 myoblasts differentiation. Biochem Biophys Res Commun 2015;467:670-5. [Crossref] [PubMed]
- Kummer D, Ebnet K. Junctional Adhesion Molecules (JAMs): The JAM-Integrin Connection. Cells 2018;7. [Crossref] [PubMed]


