
“For your eyes only”: ophthalmic complications following lung transplantation
Introduction
Lung transplantation has witnessed significant growth over the past decade, with as many as 4,122 adult transplants performed worldwide in 2015 (1). With the increasing donor pool, the advent of improved immunosuppressive drugs and surgical techniques, this number is expected to increase significantly in the coming years. Lifelong immunosuppression in these recipients makes them subject to a host of complications affecting multiple organ systems. Ophthalmic complications after lung transplantation are uncommon and range from infections to malignancies (Table 1). Some side effects are specific to the drug such as cyclosporine (CsA) induced retinopathy, while others can be attributed to systemic immunosuppression. The authors provide a detailed review of the various ophthalmic complications after lung transplantation with emphasis on early recognition of symptoms and emerging treatment options.
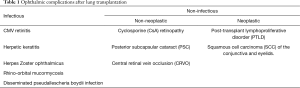
Full table
Infections
Cytomegalovirus (CMV) retinitis
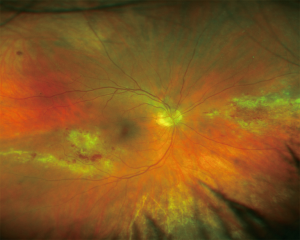
CMV retinitis is a potentially sight-threatening infection caused by CMV, a deoxyribonucleic acid (DNA) virus of the herpesviridae family. It can be established as a new infection in seronegative recipients or from reactivation of latent disease. The virus can also be transmitted to the recipient from the transplanted lungs. Serological mismatch between a seropositive donor and a seronegative recipient is the biggest risk factor for CMV infection. Another risk factor is the use of lymphocyte-depleting antibodies, such as Basiliximab, as part of anti-rejection induction therapy. The incidence of CMV infection in lung or heart-lung transplant patients is higher compared to other solid organ transplants (SOTs); 50–75% versus 22–29% after liver and 8–32% after kidney transplantation (2,3).
Patients usually present with floaters or reduced vision with the latter occasionally progressing to painless total loss of vision. Fundoscopy reveals yellow-white retinal infiltrates, retinal hemorrhages and signs of retinal vasculitis (Figure 1). The diagnosis can be made on the basis of a detailed history and fundoscopic examination. CMV viral load quantification can be performed using polymerase chain reaction (PCR). Monitoring CMV viral load over time can be more important in predicting disease rather than the absolute viral load.
Prophylaxis with oral valganciclovir is recommended for a minimum of 100 days with consideration for longer periods of up to 6 months (4). Periodic testing for CMV viremia—every 2 weeks for the first six months and monthly from 6 to 12 months—is also part of the protocol. Chmiel et al. showed a reduction in the incidence of CMV related disease from 75% (6/8) to 11% (11/96) in patients receiving antiviral prophylaxis with ganciclovir or valganciclovir (5).
Treatment for CMV retinitis varies based on its severity and resistance patterns and consists of induction with intravenous ganciclovir (5 mg/kg) twice a day for 2–3 weeks followed by maintenance therapy. Valganciclovir has emerged as an effective alternative in patients relapsing on ganciclovir therapy due to its higher bioavailability. Antiviral drug resistance can develop with prolonged treatment. Ganciclovir-resistance is due to mutations in the drug target protein kinases UL97 and UL54. Foscarnet is reserved for ganciclovir-resistant cases. Resistance to both oral valganciclovir and systemic and intravitreal foscarnet can be successfully overcome by addition of leflunomide (6). An experimental drug, AIC 246, now called letermovir, which acts on the terminase complex, in conjunction with reduced immunosuppressive therapy was successfully used to treat a multidrug-resistant CMV disease involving the lungs, gastrointestinal tract and retina (7). With a growing number of resistant strains, newer and targeted therapies towards CMV, such as adaptive T-cell therapy are being tested.
Herpetic keratitis
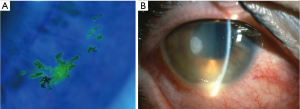
Herpetic keratitis is a viral infection of the cornea caused by the herpes simplex virus (HSV), a DNA virus from the herpesviridae family. It is estimated that around 500,000 people are infected with ocular HSV in the United States (8). After primary infection has been established through the mucous membranes, the virus establishes latency in the trigeminal ganglia. This property of the virus is responsible for recurrent infections, particularly in the immunosuppressed population. Transplant recipients are particularly at risk of reactivation during the first month after surgery. Epithelial keratitis is the most common subtype of the infection. Infection is typically unilateral and presents with redness, watery discharge, irritation, pain, and photophobia (9). Visualization of a typical dendrite is carried out on a slit-lamp examination aided with fluorescein dye (Figure 2A). Slit-lamp examination may also reveal corneal edema, keratic precipitates and hypopyon (Figure 2B). Atypical corneal lesions described as non-dendritic or geographical ulcers with terminal bulbs and epithelial infiltrations may be seen. In such cases, diagnosis can be made using PCR (10). Infection with other viruses like varicella zoster, adenovirus, enterovirus, amebic or fungal infections can mimic herpetic keratitis.
Treatment of herpetic keratitis involves topical and systemic acyclovir. Mutation in viral thymidine kinase, which is essential for phosphorylation of acyclovir to its active form, is the most common cause of resistance (11). Turner et al. reported a case of acyclovir-resistant herpetic keratitis in a patient with cystic fibrosis, post bilateral lung transplantation, who was successfully treated by addition of trifluridine eye drops to oral valacyclovir (12). Intravenous foscarnet is the drug of choice based on other case reports of acyclovir-resistant cases (13,14). Other treatment options include cidofovir, vidarabine, and interferon. Some cases seem to occur after a prolonged period of time, even years, indicating a possible association with duration of immunosuppression as hypothesized by Ng et al. (15). Complications of the condition include uveitis, corneal plaques, and acute retinal necrosis (ARN).
Prevention is possibly the best treatment. It is important to test for HSV IgG serostatus of the recipient prior to transplantation. Seropositive patients are at a higher risk of reactivation if they do not receive prophylaxis (16). Many transplant recipients receive CMV prophylaxis in the form of oral valganciclovir, which is also approved for HSV prophylaxis. Patients who are not on CMV prophylaxis should receive acyclovir. Seronegative patients can acquire the infection through asymptomatic intimate contact. Patients should be educated appropriately as the use of protection during sex may help prevent transmission (16).
Herpes zoster ophthalmicus
Herpes zoster ophthalmicus is caused by reactivation of the varicella-zoster virus (VZV). The virus lies dormant in the sensory ganglia after primary infection, usually during childhood. Factors that can trigger reactivation include advanced age, immunosuppression and hematological malignancies such as leukemia and lymphomas. Reduced cell-mediated immunity is thought to play a role as well. In a review of 314 heart transplant recipients, 51 developed herpes zoster including 14% with eye involvement and 45% with postherpetic neuralgia (17).
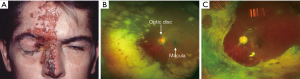
The disease presentation is similar among immunocompetent and immunocompromised individuals, while disease duration and severity may be longer and more severe in the latter group. Herpes zoster can involve eyelids, presenting as vesicular lesions, which can become pustular and/or hemorrhagic (Figure 3A). They form crusts in 7–10 days at which point the virus is not transmissible. Patients are prone to developing secondary bacterial infections like Staphylococcus aureus.Corneal involvement includes various types of keratitis. The punctate type of keratitis can coalesce to form a pseudodendritic pattern. Differentiation between herpes simplex and zoster is key as herpes simplex lesions can worsen with steroids. Steroids are not recommended in the immunocompromised population as they may cause dissemination of the virus unless there is severe involvement. Conjunctival involvement could also occur in a papillary or follicular pattern. Patients with uveitis may exhibit pain and/or photophobia. Its neurologic sequelae include extraocular muscle palsies, Horner’s syndrome, optic neuritis, retrobulbar neuritis and ischemic optic retinopathy. Retinal involvement can occur in the form of retinal vasculitis, ARN, and progressive outer retinal necrosis syndrome. ARN is characterized by necrotizing retinitis and usually panuveitis (Figure 3B,C). Postherpetic neuralgia is a common complication which may present as burning or lancinating pain, allodynia, with or without sensory deficits. Herpes zoster ophthalmicus should be considered in transplant patients presenting with changes in visual acuity, headaches, cranial nerve dysfunction and a pustular skin rash.
Management involves initial intravenous acyclovir followed by an oral regimen. Intravitreal drugs may also be required (18). A tricyclic antidepressant amitryptiline, is considered the first line treatment for postherpetic neuralgia (19).
There is insufficient evidence to support long term use of prophylactic antivirals in SOT recipients (18). Prevention with varicella vaccine (live attenuated Oka vaccine) should be given to susceptible patients prior to the transplantation (18).
Rhino-orbital mucormycosis
Rhino-orbital mucormycosis (zygomycosis) is a potentially devastating invasive fungal infection (IFI) caused by fungi belonging to the genera Mucor, Rhizopus, Rhizomucor and Absidia. Incidence of mucormycosis in SOT recipients ranges from 0.4–16% (20). Mucormycosis comprised 2% of all IFIs, as reported in the Transplant-Associated Infection Surveillance Network (TRANSNET) data (21). Renal failure, diabetes mellitus, and prior voriconazole and/or caspofungin use were associated with a higher risk of mucormycosis in SOT recipients (22). Tacrolimus use has been associated with a reduced risk for mucormycosis over cyclosporine (22). Iron overload along with the iron chelator deferoxamine have also been associated with increased risk (23,24).
Infection in humans is established by inhalation of spores and its subsequent proliferation in the nasal sinuses. The fungi invade the blood vessels leading to thrombosis and tissue necrosis. Intraocular spread occurs from invasion of the lamina papyracea. Infection can also spread through the ethmoidal and orbital veins to the cavernous sinus.
Patients can present with bloody nasal discharge, facial pain, fever, headache, decreased visual acuity, orbital edema, proptosis, ophthalmoplegia or diplopia depending on site of involvement (Figure 4A). Palatal ulceration due to maxillary spread and cranial nerve involvement can also be seen (Figure 4B).
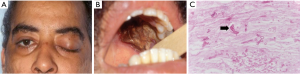
Magnetic resonance imaging (MRI) and computed tomography (CT) of the involved area is useful in assessing the spread of the disease. Negative imaging studies, however, do not rule out infection. Cultures from the sinuses, nasal discharge and blood rarely confirm the diagnosis and may be contaminated with nasal flora. Rapid diagnostic tests like PCR have been used but there are no standardized tests currently recommended. Hadaschik et al. reported the detection of Rhizopus pusillus using a commercially available panfungal PCR assay (26). The diagnosis requires a high degree of suspicion due to its similarities with other filamentous fungi. Histological examination may demonstrate presence of wide, aseptate or poorly septated ribbon-like hyphae (Figure 4C).
Treatment involves an aggressive multidisciplinary approach. Liposomal amphotericin and amphotericin lipid complex are the drugs of choice and should be started empirically in cases with strong clinical suspicion. Surgical debridement of the affected tissue is key. Reduction of immunosuppression should be considered along with correction of ketoacidosis and hyperglycemia. The use of granulocyte colony-stimulating factor (G-CSF) and granulocyte-monocyte colony stimulating factor (GM-CSF) has been advocated in neutropenic patients. Posaconazole has been used for salvage therapy in many cases with good results (27). Despite advances in surgical and medical therapies, morbidity and mortality from the condition remain high. The infection can be fatal if not treated early and aggressively. Sun et al. found an overall mortality of 52.3% (46/88) in SOT recipients with rhino-orbital-cerebral mucormycosis. The mortality in patients with CNS disease was 73.5% (36/49) (28).
Pseudallescheria boydii infection
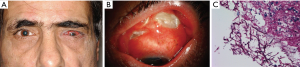
Pseudallescheria boydii or Scedosporium apiospermum (Sca) is a ubiquitous, filamentous fungi commonly found in the soil and stagnant or polluted water. They are rare causes of opportunistic infection in the immunocompromised population including SOT recipients. Risk factors include neutropenia and steroid use. Transmission occurs via inhalation through the lungs, paranasal sinuses or traumatic inoculation of the skin. Cimon et al. reported that 11/128 (8.6%) sputum samples are positive for Sca in cystic fibrosis patients (29). Disseminated infection can be life-threatening and involve the skin, heart, lung, bone, eye and central nervous system. Presenting symptoms vary depending on the organ involved. Patients may present with fever, intractable headache, lung involvement or neurological complaints like hemiplegia, aphasia, decreased visual acuity and eye pain.
Diagnosis is made by histopathological examination of bronchoalveolar lavage (BAL) culture, sputum, pleural fluid, and radiology. Microscopically, the organism appears as septate hyphae with acute angles of branching. Its resemblance to Aspergillus and Fusarium species in terms of clinical presentation and histopathological examination makes the diagnosis challenging (Figure 5A,B,C). Molecular techniques of DNA sequencing and random amplification of polymorphic DNA (RAPD) genotyping is used for species identification. This is particularly important as most infections with Sca are resistant to amphotericin B. Voriconazole is widely considered as the drug of choice (32). Some cases have been treated with the addition of echinocandins and/or terbinafine (33). Surgical debridement of the paranasal sinuses and additional local voriconazole therapy to prevent invasive disease has also been suggested (33). Surgical intervention with debridement and keratoplasty is commonly used alongside medical therapy. Evisceration is needed in many cases. Mortality for disseminated infection in SOT recipients remains extremely high (34).
Non-infectious complications
Non-neoplastic
Cyclosporine-induced ocular toxicity
Cyclosporine (CsA), used as part of the immunosuppressive regimen in lung transplant patients, inhibits transcription of interleukin-2 (IL-2) in T cells, thereby preventing the proliferation of activated T cells. Owing to its narrow therapeutic window and high variability in absorption rates, CsA levels need to be carefully monitored. High cyclosporine levels have shown to cause ocular toxicity. CsA-induced neurotoxicity is also well documented and the occipital white matter seems to be uniquely susceptible (35). CsA-related ocular toxicity can vary from optic disc edema to retinopathy and in some cases cortical blindness (36).
Patients can present with blurry vision or visual field defects months to years after starting therapy. The average latency for development of optic disc edema and retinopathy in BMT recipients is approximately 150 days (37). Fundoscopy is essential for the diagnosis. Cotton wool spots, bilateral optic disc edema, retinal hemorrhages, lipid deposits, retinal edema, macular edema, and ischemic vasculopathy are all possible findings on examination (Figure 6). Evaluation of cyclosporine-induced cortical blindness with the help of an MRI has also been reported (39).
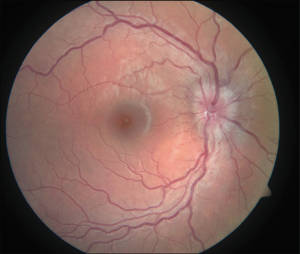
Acetazolamide has been reported to successfully treat CsA induced bilateral disc swelling in lung transplant patients (15). Discontinuing or reducing the CsA dose is adequate in most cases with complete resolution in a few weeks (40,41). Patients with cortical blindness show a more immediate response after withdrawal of cyclosporine.
Central retinal vein occlusion (CRVO)
CRVO is the second most common retinal vascular disorder after diabetic retinopathy (42). It can be ischemic or non-ischemic depending on the areas of perfusion. Ischemia can lead to complications like macular edema and neovascularization of the retina. Risk factors include advanced age, hypertension, diabetes and hyperlipidemia (43). Hypercoagulable states such as low plasma Antithrombin III levels, elevated fibrinogen levels, factor V Leiden mutation resulting in active protein C resistance along with protein C and S deficits, elevated factor VIII and IX levels have also been described in the literature as risk factors in the general population with limited information regarding the transplant population (44-47). Post-transplant immunosuppressive drugs such as cyclosporine and tacrolimus increase platelet aggregation in response to physiologic agonists and are postulated to increase the risk of CRVO in this subpopulation.
Patients typically present with sudden loss or blurring of vision. Fundoscopy reveals dilation of the retinal veins, tortuosity or intraretinal hemorrhages (Figure 7). Macular edema is a common finding at the time of diagnosis. Fundus fluorescein angiography helps to differentiate between ischemic and nonischemic CRVO. In a systematic review by McIntosh et al., the authors reported resolution of symptoms within 15 months in around 30% of nonischemic cases and 73% of ischemic cases of CRVO (48).
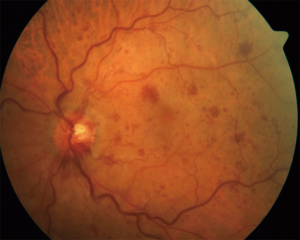
Treatment options include the following: intraocular ranibizumab, an antibody against vascular endothelial growth factor antibody (anti-VEGF), is effective for reducing macular edema and improving the vision (49). Intravitreal steroid injections or implants along with laser photocoagulation have also been found to be effective (50-52).
Posterior subcapsular cataract (PSC)
Subcapsular cataracts often develop in the posterior cortical layer of the lens among SOT recipients. They are more common in younger patients compared to the nuclear and cortical cataracts in the general population. In the transplant population, this is a common complication of prolonged steroid use. Black et al. was the first to notice the relationship between steroid use and cataract formation in rheumatoid arthritis patients in 1960; 17 (39%) of his 44 patients developing PSC (53). Many different mechanisms have been proposed in the formation of PSC, including oxidative damage, role of the glucocorticoid receptor and defective differentiation due to dysregulation of apoptosis (54). PSC has also been related to the advancing age. These factors lead to the high incidence in the transplant population. Use of even a single dose of intravitreal steroids has been shown to induce cataract (55,56). Both topical, as well as inhaled steroids, have been shown to induce cataract (57,58).
Tarabishy et al. reported the finding in 13 (28.3%) out of 46 lung transplant recipients, making it the most common ophthalmologic complication in this population (59). Data from other SOT recipients show a similar trend. Debnath et al. reported an incidence of 32.8% among 61 renal transplant patients (60). After 15 years, the incidence plateaued. Multivariate analysis showed age, body mass index (BMI) and cumulative dose of steroids as independent risk factors. Use of cyclosporine has been shown to increase the incidence of steroid-induced cataract (61).
Glare and reduced visual acuity are the most common symptoms. Slit-lamp examination shows a sheen on the posterior cortical layers early on followed by granular and plaque-like opacities (Figure 8).
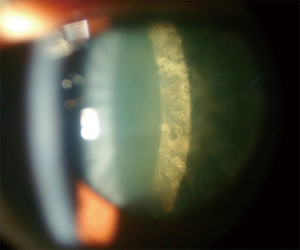
Treatment involves surgical removal and replacement of the lens as in other forms of cataract. In children, withdrawal of steroids early on may occasionally result in resolution of changes (62).
Neoplastic
Post-transplant lymphoproliferative disorder (PTLD)
PTLD is a term that encompasses a wide array of disorders due to B lymphocyte proliferation after organ transplantation. It can range from benign lymphoid hyperplasia to highly aggressive lymphomas. Intraocular involvement is a rare manifestation of PTLD. However, central nervous system involvement is common with Penn reporting an incidence of 28% (63).
Epstein-Barr virus (EBV) is thought to be the causative organism. An imbalance between B cell stimulation and anti-EBV response leads to the disease, in most instances. Chronic antigenic stimulation from the allograft, oncogenic potential of EBV and immunosuppression result in B lymphocyte proliferation (64-67). In some cases, there is monoclonal proliferation, which may result in malignancy. The degree of immunosuppression is a major risk factor, with higher levels portending an increased risk (64,68). Use of cyclosporine, in particular, has been found to be associated with many cases of ocular PTLD (69).
Posterior segment of the eye is frequently involved including the retina, vitreous and optic nerve. Tumor cells in the vitreous may mimic uveitis. Uvea may also be affected particularly in patients with systemic lymphoma. Patients present with decreased vision, floaters, photosensitivity or may even be asymptomatic. Bilateral involvement is a common occurrence. On examination, anterior chamber cells, varying degrees of flare, and iris nodules are seen in almost all patients. Posterior chamber involvement in the form of a vitreous haze and retinal involvement may also be seen (Figure 9A,B,C,D). The differential diagnosis includes toxoplasmosis, CMV, and bacterial infections. Measurements of EBV antibody titers, nuclear and capsid antigens are used to confirm clinical suspicion. Biopsy of the choroid and vitreous are other diagnostic options.
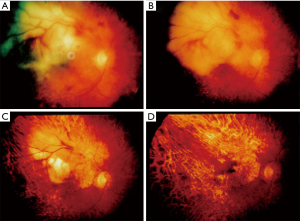
Treatment for non-clonal PTLD involves a reduction in the dose of the immunosuppression and use of antivirals like acyclovir (65,66). There may be a period of months where there is no improvement and even clinical worsening before improvement. Patients with only lymphoid hyperplasia may not need a reduction in the immunosuppressive dose. Those with true lymphoma (stage III) may not improve even with immunosuppressive dose reduction. They are treated with radiation therapy, chemotherapy, surgery and discontinuation of immunosuppression if possible (65).
Squamous cell carcinoma (SCC) of the conjunctiva and eyelids
SCC of the eye primarily involves the conjunctiva, cornea and eyelids (Figure 10). In HIV patients, the standardized incidence ratio (SIR) which measures excess risk was 12.2 for conjunctival SCC (71). Incidence in the SOT populations has been reported to be 65–250 times more than the general population for cutaneous SCC (72,73). Incidence increases with the duration of immunosuppression (72). Risk factors include ultraviolet (UV) light exposure, human papillomavirus (HPV) infection and prior skin cancers (74-76). Individual drugs increase its risk by different mechanisms. Calcineurin inhibitors act by increasing transforming growth factor B and VEGF (77). Tacrolimus and mycophenolate mofetil compromise the proper response to UV-B induced apoptosis and UV-B induced checkpoint signalling (78). In addition, SCC is much more aggressive in the immunocompromised population, including lung transplant recipients.
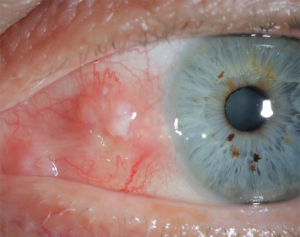
Patients with conjunctival SCC usually have a pinkish, fleshy appearing mass close to or involving the limbus. Eyelid involvement is also seen, with lower eyelid involvement more common. Visual acuity remains unchanged in most cases. Aggressive tumors may show extension to the neighboring periocular structures, lymph nodes and even the brain. Biopsy of the suspected tissue is used for diagnostic purposes showing squamous cells.
Treatment includes surgical excision with clear margins whenever possible. Moh's micrographic technique has been used in some cases (76). Invasive tumors require extensive surgical procedures including use of skin flaps, parotidectomy, cheek lift and even exenteration (76,79). Reduction in immunosuppression may be useful, but it must be balanced with the risk of rejection. Additional treatment with topical therapy like interferon eye drops (1 million units/mL) and mitomycin (0.04%) drops has been used (80).
Conclusions
Ophthalmic complications following lung transplantation although rare can be sight-threatening and potentially life-threatening if left unchecked. These conditions require a high degree of suspicion for early diagnosis and treatment. Use of antiviral prophylaxis helps to reduce the incidence of CMV retinitis and herpetic keratitis and hence form an integral part of treatment protocols. Treatment of infectious complications and cyclosporine toxicity may need reduction or discontinuation of immunosuppression. Adjunctive and alternative treatments should be pursued in cases that do not respond adequately. An ophthalmologist should urgently evaluate all patients with ocular symptoms. Although there are guidelines for ocular screening in hematopoietic cell transplants recipients, no such recommendations exist for lung transplant patients (81). There have been conflicting data to incorporate routine ocular screening in SOT including lung transplant recipients (82,83). Future research should look into the utility of scheduled ocular screening at regular intervals with the possibility of incorporating it into existing guidelines.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chambers DC, Yusen RD, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J Heart Lung Transplant 2017;36:1047-59. [Crossref] [PubMed]
- Simon DM, Levin S. Infectious complications of solid organ transplantations. Infect Dis Clin North Am 2001;15:521-49. [Crossref] [PubMed]
- Azevedo LS, Pierrotti LC, Abdala E, et al. Cytomegalovirus infection in transplant recipients. Clinics 2015;70:515-23. [Crossref] [PubMed]
- Zamora MR, Davis RD, Leonard C. Management of cytomegalovirus infection in lung transplant recipients: evidence-based recommendations. Transplantation 2005;80:157-63. [Crossref] [PubMed]
- Chmiel C, Speich R, Hofer M, et al. Ganciclovir/valganciclovir prophylaxis decreases cytomegalovirus-related events and bronchiolitis obliterans syndrome after lung transplantation. Clin Infect Dis 2008;46:831-9. [Crossref] [PubMed]
- Rifkin LM, Minkus CL, Pursell K, et al. Utility of Leflunomide in the Treatment of Drug Resistant Cytomegalovirus Retinitis. Ocul Immunol Inflamm 2017;25:93-6. [Crossref] [PubMed]
- Kaul DR, Stoelben S, Cober E, et al. First Report of Successful Treatment of Multidrug-Resistant Cytomegalovirus Disease with the Novel Anti-CMV Compound AIC246. Am J Transplant 2011;11:1079-84. [Crossref] [PubMed]
- Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol 2012;57:448-62. [Crossref] [PubMed]
- Darougar S, Wishart MS, Viswalingam ND. Epidemiological and clinical features of primary herpes simplex virus ocular infection. Br J Ophthalmol 1985;69:2-6. [Crossref] [PubMed]
- Koizumi N, Nishida K, Adachi W, et al. Detection of herpes simplex virus DNA in atypical epithelial keratitis using polymerase chain reaction. Br J Ophthalmol 1999;83:957-60. [Crossref] [PubMed]
- Duan R, de Vries RD, van Dun JM, et al. Acyclovir susceptibility and genetic characteristics of sequential herpes simplex virus type 1 corneal isolates from patients with recurrent herpetic keratitis. J Infect Dis 2009;200:1402-14. [Crossref] [PubMed]
- Turner LD, Beckingsale P. Acyclovir-resistant herpetic keratitis in a solid-organ transplant recipient on systemic immunosuppression. Clin Ophthalmol 2013;7:229-32. [Crossref] [PubMed]
- Choong K, Walker NJ, Apel AJ, et al. Aciclovir-resistant herpes keratitis. Clin Exp Ophthalmol 2010;38:309-13. [PubMed]
- Safrin S, Crumpacker C, Chatis P, et al. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med 1991;325:551-5. [Crossref] [PubMed]
- Ng P, McCluskey P, McCaughan G, et al. Ocular complications of heart, lung, and liver transplantation. Br J Ophthalmol 1998;82:423-8. [Crossref] [PubMed]
- Wilck MB, Zuckerman RA, Infectious Diseases AST. Community of Practice. Herpes Simplex Virus in Solid Organ Transplantation. Am J Transplant 2013;13:121-7. [Crossref] [PubMed]
- Koo S, Gagne LS, Lee P, et al. Incidence and risk factors for herpes zoster following heart transplantation. Transpl Infect Dis 2014;16:17-25. [Crossref] [PubMed]
- Pergam SA, Limaye AP. Practice ASTIDCo. Varicella zoster virus (VZV) in solid organ transplant recipients. Am J Transplant 2009;9 Suppl 4:S108-15. [Crossref] [PubMed]
- Wu CL, Raja SN. An update on the treatment of postherpetic neuralgia. J Pain 2008;9:S19-30. [Crossref] [PubMed]
- Lanternier F, Sun HY, Ribaud P, et al. Mucormycosis in Organ and Stem Cell Transplant Recipients. Clin Infect Dis 2012;54:1629-36. [Crossref] [PubMed]
- Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010;50:1101-11. [Crossref] [PubMed]
- Singh N, Aguado JM, Bonatti H, et al. Zygomycosis in solid organ transplant recipients: a prospective, matched case-control study to assess risks for disease and outcome. J Infect Dis 2009;200:1002-11. [Crossref] [PubMed]
- Sugar AM. Mucormycosis. Clin Infect Dis 1992;14 Suppl 1:S126-9. [Crossref] [PubMed]
- Maertens J, Demuynck H, Verbeken EK, et al. Mucormycosis in allogeneic bone marrow transplant recipients: report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant 1999;24:307-12. [Crossref] [PubMed]
- Nieto-Ríos JF, Moreno-Coral LF, Zapata-Cardenas A, et al. Successful treatment of rhino-orbital-cerebral mucormycosis in a kidney transplant patient. Nefrologia 2014;34:120-4. [PubMed]
- Hadaschik E, Koschny R, Willinger B, et al. Pulmonary, rhino-orbital and cutaneous mucormycosis caused by Rhizomucor pusillus in an immunocompromised patient. Clin Exp Dermatol 2012;37:355-7. [Crossref] [PubMed]
- Arda B, Erdem A, Sipahi OR, et al. Mucormycosis: retrospective evaluation of 12 cases. Mikrobiyol Bul 2011;45:504-11. [PubMed]
- Sun HY, Forrest G, Gupta KL, et al. Rhino-orbital-cerebral zygomycosis in solid organ transplant recipients. Transplantation 2010;90:85-92. [Crossref] [PubMed]
- Cimon B, Carrere J, Vinatier JF, et al. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis 2000;19:53-6. [Crossref] [PubMed]
- Law JC, Breazzano MP, Eliott D. Scleral Buckle Infection With Pseudallescheria boydii. Ophthalmic Surg Lasers Imaging Retina 2017;48:676-8. [Crossref] [PubMed]
- Balandin B, Aguilar M, Sánchez I, et al. Scedosporium apiospermum and S. prolificans mixed disseminated infection in a lung transplant recipient: An unusual case of long-term survival with combined systemic and local antifungal therapy in intensive care unit. Med Mycol Case Rep 2016;11:53-6. [Crossref] [PubMed]
- Gilgado F, Serena C, Cano J, et al. Antifungal susceptibilities of the species of the Pseudallescheria boydii complex. Antimicrob Agents Chemother 2006;50:4211-3. [Crossref] [PubMed]
- Hartmann C, Müller C, Weißbrodt H, et al. Successful prevention of scedosporiosis after lung transplantation in a cystic fibrosis patient by combined local and systemic triazole therapy. Med Mycol Case Rep 2013;2:116-8. [Crossref] [PubMed]
- Castiglioni B, Sutton DA, Rinaldi MG, et al. Pseudallescheria boydii (Anamorph Scedosporium apiospermum). Infection in solid organ transplant recipients in a tertiary medical center and review of the literature. Medicine (Baltimore) 2002;81:333-48. [Crossref] [PubMed]
- Bechstein WO. Neurotoxicity of calcineurin inhibitors: impact and clinical management. Transpl Int 2000;13:313-26. [Crossref] [PubMed]
- Reece DE, Frei-Lahr DA, Shepherd JD, et al. Neurologic complications in allogeneic bone marrow transplant patients receiving cyclosporin. Bone Marrow Transplant 1991;8:393-401. [PubMed]
- Coskuncan NM, Jabs DA, Dunn JP, et al. The eye in bone marrow transplantation. VI. Retinal complications. Arch Ophthalmol 1994;112:372-9. [Crossref] [PubMed]
- Kalinina Ayuso V, Hettinga Y, van der Does P, et al. Ocular complications in children within 1 year after hematopoietic stem cell transplantation. JAMA Ophthalmol 2013;131:470-5. [Crossref] [PubMed]
- Monteiro L, Almeida-Pinto J, Rocha N, et al. Case report: cyclosporin A-induced neurotoxicity. Br J Radiol 1993;66:271-2. [Crossref] [PubMed]
- López-Jiménez J, Sanchez A, Fernandez CS, et al. Cyclosporine-induced retinal toxic blindness. Bone Marrow Transplant 1997;20:243-5. [Crossref] [PubMed]
- O'Riordan JM, FitzSimon S, O'Connor M, et al. Retinal microvascular changes following bone marrow transplantation: the role of cyclosporine. Bone Marrow Transplant 1994;13:101-4. [PubMed]
- Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol 2006;124:726-32. [Crossref] [PubMed]
- Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol 2001;131:61-77. [Crossref] [PubMed]
- Izbicki G, Bairey O, Shitrit D, et al. Increased thromboembolic events after lung transplantation. Chest 2006;129:412-6. [Crossref] [PubMed]
- Lahey JM, Tunc M, Kearney J, et al. Laboratory evaluation of hypercoagulable states in patients with central retinal vein occlusion who are less than 56 years of age. Ophthalmology 2002;109:126-31. [Crossref] [PubMed]
- Johnson TM, El-Defrawy S, Hodge WG, et al. Prevalence of factor V Leiden and activated protein C resistance in central retinal vein occlusion. Retina 2001;21:161-6. [Crossref] [PubMed]
- Bertram B, Remky A, Arend O, et al. Protein C, protein S, and antithrombin III in acute ocular occlusive diseases. Ger J Ophthalmol 1995;4:332-5. [PubMed]
- McIntosh RL, Rogers SL, Lim L, et al. Natural history of central retinal vein occlusion: an evidence-based systematic review. Ophthalmology 2010;117:1113-1123.e15. [Crossref] [PubMed]
- Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1124-1133.e1. [Crossref] [PubMed]
- Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs. Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol 2009;127:1101-14. [Crossref] [PubMed]
- Haller JA, Bandello F, Belfort R Jr, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010;117:1134-1146.e3. [Crossref] [PubMed]
- Pikkel YY, Sharabi-Nov A, Beiran I, et al. Comparison of anti-vascular endothelial growth factors, laser treatments and a combination of the both for treatment of central retinal vein occlusion. Int J Ophthalmol 2016;9:431-3. [PubMed]
- Black RL, Oglesby RB, Von Sallmann L, et al. Posterior subcapsular cataracts induced by corticosteroids in patients with rheumatoid arthritis. JAMA 1960;174:166-71. [Crossref] [PubMed]
- Petersen A, Carlsson T, Karlsson JO, et al. Effects of dexamethasone on human lens epithelial cells in culture. Mol Vis 2008;14:1344-52. [PubMed]
- Cekiç O, Chang S, Tseng JJ, et al. Cataract progression after intravitreal triamcinolone injection. Am J Ophthalmol 2005;139:993-8. [Crossref] [PubMed]
- Jonas JB, Degenring R, Vossmerbauemer U, et al. Frequency of cataract surgery after intravitreal injection of high-dosage triamcinolone acetonide. Eur J Ophthalmol 2005;15:462-4. [Crossref] [PubMed]
- Gasset AR, Bellows RT. Posterior subcapsular cataracts after topical corticosteroid therapy. Ann Ophthalmol 1974;6:1263-5. [PubMed]
- Fraunfelder FT, Meyer SM. Posterior subcapsular cataracts associated with nasal or inhalation corticosteroids. Am J Ophthalmol 1990;109:489-90. [Crossref] [PubMed]
- Tarabishy AB, Khatib OF, Nocero JR, et al. Ocular complications in patients with lung transplants. Br J Ophthalmol 2011;95:1295-8. [Crossref] [PubMed]
- Debnath SC, Abomelha MS, Jawdat M, et al. Ocular side effects of systemic steroid therapy in renal transplant patients. Ann Ophthalmol 1987;19:435-7. [PubMed]
- Nakamura T, Sasaki H, Nagai K, et al. Influence of cyclosporin on steroid-induced cataracts after renal transplantation. Jpn J Ophthalmol 2003;47:254-9. [Crossref] [PubMed]
- Forman AR, Loreto JA, Tina LU. Reversibility of corticosteroid-associated cataracts in children with the nephrotic syndrome. Am J Ophthalmol 1977;84:75-8. [Crossref] [PubMed]
- Penn I. The changing pattern of posttransplant malignancies. Transplant Proc 1991;23:1101-3. [PubMed]
- Penn I. Cancers complicating organ transplantation. N Engl J Med 1990;323:1767-9. [Crossref] [PubMed]
- Hanto DW, Gajl-Peczalska KJ, Frizzera G, et al. Epstein-Barr virus (EBV) induced polyclonal and monoclonal B-cell lymphoproliferative diseases occurring after renal transplantation. Clinical, pathologic, and virologic findings and implications for therapy. Ann Surg 1983;198:356-69. [Crossref] [PubMed]
- Nalesnik MA, Jaffe R, Starzl TE, et al. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol 1988;133:173-92. [PubMed]
- Cockfield SM, Preiksaitis JK, Jewell LD, et al. Post-transplant lymphoproliferative disorder in renal allograft recipients. Clinical experience and risk factor analysis in a single center. Transplantation 1993;56:88-96. [Crossref] [PubMed]
- Penn I. Why do immunosuppressed patients develop cancer? Crit Rev Oncog 1989;1:27-52. [PubMed]
- Cho AS, Holland GN, Glasgow BJ, et al. Ocular involvement in patients with posttransplant lymphoproliferative disorder. Arch Ophthalmol 2001;119:183-9. [PubMed]
- Demols PF, Cochaux PM, Velu T, et al. Chorioretinal post-transplant lymphoproliferative disorder induced by the Epstein-Barr virus. Br J Ophthalmol 2001;85:93. [Crossref] [PubMed]
- Guech-Ongey M, Engels EA, Goedert JJ, et al. Elevated risk for squamous cell carcinoma of the conjunctiva among adults with AIDS in the United States. Int J Cancer 2008;122:2590-3. [Crossref] [PubMed]
- Hartevelt MM, Bavinck JN, Kootte AM, et al. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation 1990;49:506-9. [Crossref] [PubMed]
- Jensen P, Hansen S, Moller B, et al. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J Am Acad Dermatol 1999;40:177-86. [Crossref] [PubMed]
- Lindelöf B, Sigurgeirsson B, Gabel H, et al. Incidence of skin cancer in 5356 patients following organ transplantation. Br J Dermatol 2000;143:513-9. [PubMed]
- Lee GA, Hirst LW. Retrospective study of ocular surface squamous neoplasia. Aust N Z J Ophthalmol 1997;25:269-76. [Crossref] [PubMed]
- Perry JD, Polito SC, Chundury RV, et al. Periocular Skin Cancer in Solid Organ Transplant Recipients. Ophthalmology 2016;123:203-8. [Crossref] [PubMed]
- Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol 2012;13:354-76. [Crossref] [PubMed]
- Ming M, Zhao B, Qiang L, et al. Effect of immunosuppressants tacrolimus and mycophenolate mofetil on the keratinocyte UVB response. Photochem Photobiol 2015;91:242-7. [Crossref] [PubMed]
- Shelil AE, Shields CL, Shields JA, et al. Aggressive conjunctival squamous cell carcinoma in a patient following liver transplantation. Arch Ophthalmol 2003;121:280-2. [Crossref] [PubMed]
- Shields CL, Ramasubramanian A, Mellen PL, et al. Conjunctival squamous cell carcinoma arising in immunosuppressed patients (organ transplant, human immunodeficiency virus infection). Ophthalmology 2011;118:2133-2137.e1. [Crossref] [PubMed]
- Majhail NS, Rizzo JD, Lee SJ, et al. Recommended Screening and Preventive Practices for Long-term Survivors after Hematopoietic Cell Transplantation. Bone Marrow Transplantation 2012;47:337-41. [Crossref] [PubMed]
- Tabbara KF, Qahtani FM, Wedin KL. Infections Following Organ Transplantation. Ophthalmology;116:1232-.e1.
- Akerele T, Lightman S. Ocular complications in heart, lung and heart-lung recipients. Br J Ophthalmol 2007;91:310-2. [Crossref] [PubMed]


