Fibrinogen as a potential biomarker for clinical phenotype in patients with chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a multicomponent disease comprising both lung and systemic effects that include airway remodelling, emphysema, chronic bronchitis, muscle wasting, and inflammatory response (1). This heterogeneity could also affect the prognosis and progression of the disease. Although forced expiratory volume in 1 s (FEV1) is the most commonly used marker to predict the severity and progression of the disease, airway limitation poorly correlates with symptoms or the progression of disease (2). Hence, exploring novel biomarkers for the risk stratification and assessment of therapeutic intervention is imperative.
Fibrinogen is a glycoprotein synthesized in hepatocytes and released into blood circulation (3). Fibrinogen is an essential coagulation factor, and also plays a vital role as an acute-phase reactant. Elevated plasma fibrinogen levels have been reported to be associated with a risk of COPD and other inflammatory diseases (4,5). Fibrinogen level might correlate with disease severity and exacerbation risk (6,7). Recently, United States Food and Drug Administration (FDA) and European Medicines Agency have considered plasma fibrinogen for qualification as a drug development tool (8).
Most studies on fibrinogen have been conducted in non-Asian patients with COPD; therefore, this study aims to determine whether plasma fibrinogen could be a potential biomarker of disease severity and exacerbation rate in Asian patients with COPD.
Methods
Study design and patients
The Institutional Review Board (2017-0132) of Asan Medical Center approved this retrospective study and waived the need for informed consent. We reviewed the medical records of patients treated through an outpatient clinic between January 2013 and December 2016. We enrolled patients with COPD aged >40 years, for whom fibrinogen assessment results, without acute exacerbation for the previous 3 months were available. Exclusion criteria were as follows: lack of data regarding baseline lung function; diagnose of deep vein thrombosis, pulmonary thromboembolism, disseminated intravascular coagulation or hematological malignancy, and uncontrolled malignancy or anticancer treatment; and a history of asthma or asthma-COPD overlap syndrome.
We extracted the following data: demographic characteristics, comorbidities, spirometry results, respiratory symptoms, and laboratory findings [complete blood count, and C-reactive protein (CRP) and fibrinogen levels] at the time of fibrinogen measurement. We also reviewed the history of presence and frequency of exacerbation in the previous years. Grades of COPD was determined by the percentage predicted value of FEV1 (≥80%, mild; ≥50% and <80%, moderate; ≥30% and <50%, severe; and <30%, very severe). The groups (assigned groups A, B, C, and D) were assigned based on the Global Initiative on Obstructive Lung disease, updated in 2017. Severity of COPD was calculated by three indexes: dyspnea, obstruction, smoking, exacerbation (DOSE); body mass index, airflow obstruction, dyspnea, exercise (BODE); and age, dyspnea, airflow obstruction (ADO).
Definitions
The plasma fibrinogen level was measured in patients with a stable phase of COPD. The plasma fibrinogen level was automatically evaluated per time for fibrin clot formation (Clauss assay) (9) using Dade Thrombin Reagent (Siemens Healthcare Diagnostics Products) and an automated coagulation analyzer Sysmex® CS-5100 system. In our center, the upper normal limit was defined as 400 mg/dL. However, the threshold at 350 mg/dL was applied to define the groups with high or low fibrinogen levels, as previously reported (7).
Exacerbation of COPD was defined as a composite of hospital admissions because of aggravated respiratory symptoms and/or treatment with systemic corticosteroids alone or in combination with antibiotics dispensed by pulmonologists.
Statistical analysis
We compared the study subgroup characteristics using the analysis of variance, Kruskal–Wallis test, unpaired t-test, or the Mann–Whitney U-test for continuous variables, and Fisher’s exact test for categorical variables. We tested the correlation between variables using the Spearman’s correlation coefficient and assessed clinical phenotypes of patients with high fibrinogen level by linear regression analysis. We evaluated associations of all the variables by univariate analysis; the variables considered significant (P<0.2 in univariate analysis) were then included in multivariate analysis. Five variables, including Charlson comorbidity index (CCI) score, COPD assessment test (CAT) score, spirometry finding (FEV1, percentage predicted value), distance of 6-minute walk test (6MWT), and history of acute exacerbation, were selected for risk-adjusted analysis. We conducted multivariate analysis using stepwise backward elimination method to identify independent factors associated with the fibrinogen level.
We performed statistical analyses using Statistical Package for the Social Sciences® Version 24.0 (IBM Corporation, Armonk, NY, USA). Data were expressed as mean ± standard deviation, and a P value of <0.05 was considered statistically significant.
Results
Baseline characteristics
We enrolled 155 patients in this study, but 2 hematologic and 13 non-hematologic malignancies were excluded in final analysis. Table 1 summarizes the baseline characteristics of the study population. The mean age of the patients was 70.3 (IQR: 64.5–77.0) years, 88.6% were males, and the overall mean body mass index (BMI) was 22.6 kg/m2. Overall, 121 (86.4%) patients had a smoking history, with a mean of 33.6 pack-years, and the mean CCI score was 1.9.
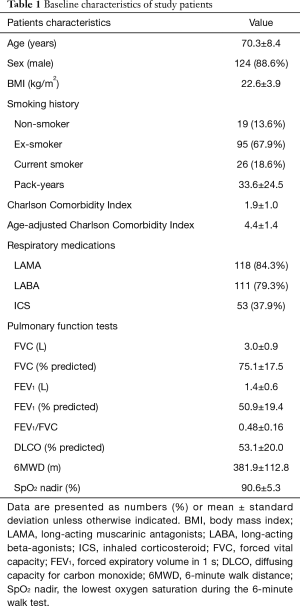
Full table
Characteristics of lung function were as follows: mean forced vital capacity (FVC), 3.0 L (75.1% predicted); FEV1, 1.4 L (50.9% predicted); and mean FEV1/FVC, 0.48. The mean diffusion capacity for carbon monoxide (DLCO) was assessed as 53.1% predicted. All patients exhibited an ability to walk for a mean of 381.9 m in the 6MWT.
Comparison between patients with low and high-level fibrinogen
Table 2 summarizes the characteristics of the patients based on the fibrinogen level. We categorized 140 patients into two groups as follows: low (92 patients, 65.7%, <350 mg/dL) and high fibrinogen level (48 patients, 34.3%, ≥350 mg/dL). Overall, the mean fibrinogen level was 327.2 (±94.7) mg/dL. The mean fibrinogen level in the low-level and high-level groups was 272.1 (±48.5) and 432.9 (±67.8) mg/dL, respectively; we observed no significant differences in the age (P=0.654), sex (P=0.994), BMI (P=0.562), smoking status (P=0.861) and smoking duration (P=0.867) between both groups.
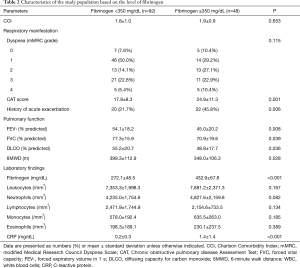
Full table
Regarding respiratory manifestation, although no significant difference was observed in the modified Medical Research Council (mMRC) dyspnea grade (P=0.115), and the high-level group had higher CAT score than the low-level group (P=0.001). The high-level group comprised more patients with medical history of acute exacerbations (P=0.006). The deterioration in lung function, including FEV1 (P=0.008), FVC (P=0.039), and distance of 6MWT (P=0.012), was significantly worse in the high-level group than in the low-level group.
Regarding laboratory findings, we observed no difference in the number of leukocytes (P=0.167), neutrophils (P=0.082), lymphocytes (P=0.134), and eosinophils (P=0.389). In particular, CRP level was elevated in the high-level group (P<0.001).
Clinical phenotype associated with fibrinogen
Table 3 shows the clinical phenotypes of COPD associated with high fibrinogen level.
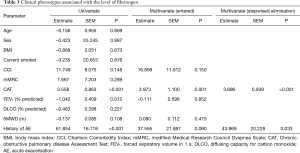
Full table
In univariate analysis, a high fibrinogen level correlated with a high CAT score (P<0.001), low FEV1 (P=0.012), and an experience of acute exacerbation (P<0.001). However, age, sex, BMI, smoking status, and dyspnea grade did not affect the fibrinogen level.
Multivariate analysis using the five variables that were significant factors in the univariate analysis revealed an elevated fibrinogen level mostly associated with CAT (P=0.001) score. Based on the multivariate analyses using the stepwise backward elimination method, this study demonstrated that the high fibrinogen level independently correlated with two factors, including poor CAT score (P<0.001), and an experience of exacerbation (P=0.033).
Correlation between the COPD severity and fibrinogen level
Table 4 and Figure 1 present the correlation between the three severity indices of COPD and fibrinogen level. The fibrinogen level demonstrated statistically significant positive correlations with DOSE (ρ=0.185, P<0.05), BODE (ρ=0.195, P<0.05), and ADO (ρ=0.175, P<0.05) indexes. The analysis revealed positive correlation of the CRP level with the BODE (ρ=0.207, P<0.05) index. However, no significant relationship was established between the leukocyte count and severity index of COPD.

Full table
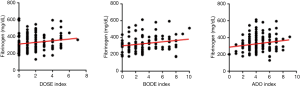
Fibrinogen level according to the COPD grade or group
Among all patients, the numbers of those with a mild, moderate, severe, and very severe grade of COPD, assessed by spirometric FEV1 were 12, 60, 43, and 23, respectively (Table 5). Fibrinogen level tended to increase with increasing grade of COPD, although statistically nonsignificant (P=0.306).
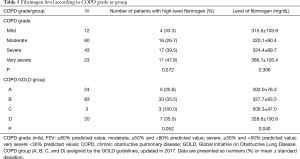
Full table
Based on the COPD groups assigned by the Global Initiative on Obstructive Lung Disease, groups A, B, C, and D, were comprised of 24, 93, 3, and 20 patients, respectively (Table 5). We observed a significant difference in the fibrinogen level among groups (P=0.040), especially in group C which had only three patients; however, the mean fibrinogen level of group C was higher than that of other groups (P<0.05).
Discussion
In Korean patients with COPD, our results revealed that the high-level fibrinogen group had more patients with history of acute exacerbation than the low-level fibrinogen group. Patients with high fibrinogen level exhibited a clinical phenotype with high CAT score, and frequent experience of acute exacerbations. The plasma fibrinogen level demonstrated a positive correlation with the COPD severity indexes, including the DOSE, BODE, and ADO.
A heterogeneous complex disease, COPD is characterized by various phenotypes, including emphysema, chronic bronchitis, and frequent exacerbation (10-12), rendering the prediction of disease progression and mortality and the application of pharmacologic agents challenging. Although some biomarkers might have become a milestone for effective therapies against COPD, the identification of biomarkers for defining the COPD subtypes or severity provides statistical power and financial benefit to clinical trials (13). At present, fibrinogen is the first biomarker drug development tool qualified for use in COPD under the FDA’s drug development tool qualification program (8); this guidance was based on five major studies, including the Atherosclerosis Risk in Communities (ARIC), Cardiovascular Health Study (CHS), Framingham Heart Study Offspring Cohort (FHSOC), National Health and Nutrition Examination Survey III (NHANES III), and Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) studies (7). Although the fibrinogen level could be affected by several demographic characteristics such as age, sex, smoking, BMI, and physical activity (4), these studies included only 0–20.7% non-Caucasians, and consequently, the results had limited applicability to Asian patients with COPD.
The mean fibrinogen level obtained in this study was lower than that obtained in the ECLIPSE study, but was similar to that in the ARIC, CHS, FHSOC, and NHANES III studies (7). This difference could be attributed to the difference in the assessment method. Several assays have been established to determine the plasma fibrinogen concentration, including the von Clauss, prothrombin time-derived, clottable protein, and immunological assays (3). Of these, the von Clauss method, assessing thrombin-clottable proteins in plasma, is the leading method in the clinical setting. However, several factors, including instrument type and reagent, could affect the results (3). In a laboratory setting, an immunological method could be used, similar to the ECLIPSE study (14). Thus, assay method standardization is warranted for fibrinogen to become a generally accepted biomarker for COPD.
As expected, the high-level group comprised more patients with history of acute exacerbation. Severe exacerbation necessitating hospitalization was reported more in the high-level group, supporting the correlation of high plasma fibrinogen level with exacerbation, and indicating that plasma fibrinogen could play an essential role as a biomarker to predict exacerbation in Asian patients with COPD. The CAT score, a promising tool to quantify COPD exacerbation, was significantly higher in the high-level group. However, some studies have demonstrated that plasma fibrinogen affected the risk and frequency of exacerbation in only a small subset of patients categorized according to the COPD severity (15-17).
Nonetheless, the correlation between fibrinogen level and disease severity remains debatable. In this study, the high-level group had more severe respiratory manifestation and exacerbation, with a higher tendency for the mMRC grade to be elevated and pulmonary functions to be deteriorated. Moreover, FEV1 significantly decreased in the high-level group than in the low-level group. Plasma fibrinogen negatively correlated with FEV1 (ρ=–0.199, P=0.019), and tended to increase with increasing grade of COPD. The FEV1 is a traditional indicator used to differentiate disease severity according to airway limitation in COPD (18-20). However, a recent study reported that an FEV1-based comparator might be ineffective in explaining the phenotype of COPD (4). Thus, we ascertained the correlation between the level of fibrinogen and severity through COPD severity indexes. The high-level group had higher DOSE, BODE, and ADO indexes (data not shown), and these values positively correlated with the fibrinogen level, suggesting a significant association between plasma fibrinogen level and COPD severity.
Fibrinogen is a known principal acute-phase reactant, and CRP is the most commonly used biomarker for systemic inflammation (21). An investigation of the relationship among three inflammatory markers, including leukocyte count, CRP level and fibrinogen level revealed a positive correlation. Especially, the CRP level significantly correlated with fibrinogen, and was elevated in the high-level group. Previous studies have reported that the combination of these three markers could enhance the predictability of all-cause mortality in patients with obstructive lung functions or exacerbations in individuals with COPD (22,23). However, a study demonstrated that fibrinogen offers advantages over CRP in terms of repeatability over time; in that study, fibrinogen was the most stable variable in the 3-month follow-up, but CRP level was the most capricious biomarker (14). Perhaps, this difference could be attributed to the different pro-inflammatory cytokines required for the activation of acute-phase reactants. Fibrinogen is released following interleukin-6 and glucocorticoid-mediated stimulation as class 2 acute-phase reactant. However, CRP, a class 1 acute-phase reactant, is primarily regulated by interleukin-1 (15). Nevertheless, additional studies are needed to firmly establish the role of fibrinogen and CRP in COPD.
Patients with COPD often exhibit comorbidities, including ischemic heart disease, peripheral vascular disease, metabolic diseases, and lung cancer (24). In these comorbidities and COPD, smoking is the most important etiology. Patients with COPD exhibiting comorbidity can be affected to a greater extent by persistent systemic inflammation and exhibit increased mortality and exacerbation rates than those without comorbidity. Hence, an indicator is required to determine the severity of systemic inflammation. Some studies have reported that patients with chronic inflammatory diseases exhibit elevated levels of fibrinogen. Hence, it is imperative to validate the correlation between the comorbidity severity and fibrinogen level. We applied CCI as a severity marker for comorbidity; and this is the most common method used to assess the 1-year mortality rate according to comorbidity (25,26). In this study, the fibrinogen level was not correlated with the CCI score (ρ=0.150, P=0.077). However, previous studies have reported fibrinogen as an independent risk factor for the development of heart disease and related mortality (27,28). On the other hand, another study insisted that inflammatory markers, including fibrinogen, were simply confounding factors related to the cause (29). In COPD, whether fibrinogen is the consequence of systemic inflammation or an independent risk factor of exacerbation and mortality remains unclear.
This study has several limitations. Firstly, this was a retrospective cross-sectional study with a small sample size, which might have affected the results or statistical power in the analysis. However, further extensive, prospective studies have already been conducted on patients of other races, except Asians. Secondly, we could not investigate the usefulness of fibrinogen in predicting mortality in this study. Hence, a well-designed prospective study in Asian patients with COPD is required to confirm the role of fibrinogen in predicting mortality. Thirdly, we did not exclude the 9 patients with past medical history of malignancy, but they had no evidence of disease for 5 years. The proportion of men among the patient in this study was higher than that in other studies. As mentioned previously, fibrinogen could be affected by various factors, including sex. Hence, further studies are warranted to confirm whether the study results are also applicable to Asian women.
Conclusions
In Korean patients with COPD, plasma fibrinogen level associated with CAT score, and history of exacerbation. The COPD severity indexes positively correlated with the plasma fibrinogen level. Hence, plasma fibrinogen could be a useful biomarker to define the clinical phenotypes in Asian patients with COPD.
Acknowledgements
This study was supported by University of Ulsan College of Medicine.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was reviewed and approved by the Institutional Review Board (2017-0132) of Asan Medical Center.
References
- Agusti AG. COPD, a multicomponent disease: implications for management. Respir Med 2005;99:670-82. [Crossref] [PubMed]
- Nishimura K, Izumi T, Tsukino M, et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002;121:1434-40. [Crossref] [PubMed]
- Undas A. How to Assess Fibrinogen Levels and Fibrin Clot Properties in Clinical Practice? Semin Thromb Hemost 2016;42:381-8. [Crossref] [PubMed]
- Duvoix A, Dickens J, Haq I, et al. Blood fibrinogen as a biomarker of chronic obstructive pulmonary disease. Thorax 2013;68:670-6. [Crossref] [PubMed]
- Valvi D, Mannino DM, Mullerova H, et al. Fibrinogen, chronic obstructive pulmonary disease (COPD) and outcomes in two United States cohorts. Int J Chron Obstruct Pulmon Dis 2012;7:173-82. [PubMed]
- Dahl M, Tybjaerg-Hansen A, Vestbo J, et al. Elevated plasma fibrinogen associated with reduced pulmonary function and increased risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1008-11. [Crossref] [PubMed]
- Mannino DM, Tal-Singer R, Lomas DA, et al. Plasma Fibrinogen as a Biomarker for Mortality and Hospitalized Exacerbations in People with COPD. Chronic Obstr Pulm Dis 2015;2:23-34. [Crossref] [PubMed]
- Miller BE, Tal-Singer R, Rennard SI, et al. Plasma Fibrinogen Qualification as a Drug Development Tool in Chronic Obstructive Pulmonary Disease. Perspective of the Chronic Obstructive Pulmonary Disease Biomarker Qualification Consortium. Am J Respir Crit Care Med 2016;193:607-13. [Crossref] [PubMed]
- Mackie IJ, Kitchen S, Machin SJ, et al. Guidelines on fibrinogen assays. Br J Haematol 2003;121:396-404. [Crossref] [PubMed]
- Vestbo J, Agusti A, Wouters EF, et al. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med 2014;189:1022-30. [Crossref] [PubMed]
- Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med 2017;5:619-26. [Crossref] [PubMed]
- Kim V, Criner GJ. The chronic bronchitis phenotype in chronic obstructive pulmonary disease: features and implications. Curr Opin Pulm Med 2015;21:133-41. [Crossref] [PubMed]
- Zemans RL, Jacobson S, Keene J, et al. Multiple biomarkers predict disease severity, progression and mortality in COPD. Respir Res 2017;18:117. [Crossref] [PubMed]
- Dickens JA, Miller BE, Edwards LD, et al. COPD association and repeatability of blood biomarkers in the ECLIPSE cohort. Respir Res 2011;12:146. [Crossref] [PubMed]
- Arellano-Orden E, Calero-Acuna C, Cordero JA, et al. Specific networks of plasma acute phase reactants are associated with the severity of chronic obstructive pulmonary disease: a case-control study. Int J Med Sci 2017;14:67-74. [Crossref] [PubMed]
- Thomsen M, Ingebrigtsen TS, Marott JL, et al. Inflammatory biomarkers and exacerbations in chronic obstructive pulmonary disease. Jama 2013;309:2353-61. [Crossref] [PubMed]
- Agustí A, Edwards LD, Rennard SI, et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One 2012;7. [Crossref] [PubMed]
- Shaw JG, Vaughan A, Dent AG, et al. Biomarkers of progression of chronic obstructive pulmonary disease (COPD). J Thorac Dis 2014;6:1532-47. [PubMed]
- Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986;133:14-20. [Crossref] [PubMed]
- Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med 2006;100:115-22. [Crossref] [PubMed]
- Bray C, Bell LN, Liang H, et al. Erythrocyte Sedimentation Rate and C-reactive Protein Measurements and Their Relevance in Clinical Medicine. WMJ 2016;115:317-21. [PubMed]
- Thomsen M, Dahl M, Lange P, et al. Inflammatory biomarkers and comorbidities in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;186:982-8. [Crossref] [PubMed]
- Ford ES, Cunningham TJ, Mannino DM. Inflammatory markers and mortality among US adults with obstructive lung function. Respirology 2015;20:587-93. [Crossref] [PubMed]
- Miller J, Edwards LD, Agusti A, et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir Med 2013;107:1376-84. [Crossref] [PubMed]
- Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57:1288-94. [Crossref] [PubMed]
- de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol 2003;56:221-9. [Crossref] [PubMed]
- Garcia-Rio F, Miravitlles M, Soriano JB, et al. Systemic inflammation in chronic obstructive pulmonary disease: a population-based study. Respir Res 2010;11:63. [Crossref] [PubMed]
- Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA 2005;294:1799-809. [PubMed]
- Fowkes FG, Anandan CL, Lee AJ, et al. Reduced lung function in patients with abdominal aortic aneurysm is associated with activation of inflammation and hemostasis, not smoking or cardiovascular disease. J Vasc Surg 2006;43:474-80. [Crossref] [PubMed]


