Extracorporeal membrane oxygenation as a rescue therapy for acute respiratory failure during chemotherapy in a patient with acute myeloid leukemia
Introduction
Acute respiratory distress syndrome (ARDS) caused by pneumonia is life-threatening, particularly in immunosuppressed patients with hematological malignancies such as acute myeloid leukemia (AML) or acute lymphoid leukemia (1-3).
Extracorporeal membrane oxygenation (ECMO) is the only temporary method that can support or replace respiratory function in patients with severe refractory respiratory failure on conventional mechanical ventilator support. However, sepsis or cytopenia after chemotherapy are relative contraindications for ECMO (4,5). Compared to standard indications for ECMO, cytopenia further increases the risks of infection and bleeding. There are some data on the use of ECMO in patients with hematological malignancies (6-8), but few reports on its use in adults with AML through chemotherapy. Here, we report a patient with pneumonia and ARDS treated with ECMO during induction chemotherapy for AML.
Case presentation
A 22-year-old man was admitted with shortness of breath and fever that started 1 week earlier. He had no noteworthy history of medical problems or recent travel or unusual food consumption.
On admission, his vital signs included a blood pressure of 140/100 mmHg, body temperature of 39.7 °C, heart rate of 100 beats/min, and respiratory rate of 35 breaths/min. Chest auscultation revealed crackles in both lower lung fields. Arterial blood gas analysis (ABGA) showed pH 7.45, pCO2 33.0 mmHg, PaO2 61.0 mmHg, HCO3 22.9 mmol/L, and SpO2 92% on oxygen supplied via nasal prong at a flow rate of 4 L/min. A complete blood count results were as follows: leukocytes 6,870/mm3 (segmented neutrophils 1.1%, lymphocytes 70.3%, monocytes 28.1%), hemoglobin 11.3 g/dL, hematocrit 32.4%, and platelets 37,000/mm3. Blood chemistry was as follows: blood urea nitrogen (BUN) 7.7 mg/dL, creatinine 0.82 mg/dL, aspartate transaminase 16 U/L, alanine transaminase 25 U/L, total bilirubin 0.81 mg/dL, lactate dehydrogenase 331 U/L, C-reactive protein 13.14 mg/dL, and procalcitonin 1.58 ng/mL. The prothrombin time (PT), international normalized ratio (INR), and activated partial thromboplastin time (aPTT) were 18.1, 1.49, and 25.8 s, respectively.
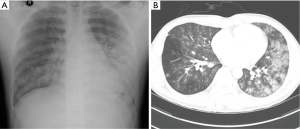
A chest radiography revealed ill-defined patchy consolidation in both lower lung field, particularly on the left side (Figure 1A). Chest computed tomography (CT) revealed bilateral ground glass opacities (GGO) with peribronchial consolidation in the both lower lobe, particularly on the left side (Figure 1B).
Empirical intravenous antibiotic therapy was initiated with cefepime and levofloxacin. An initial sputum culture was negative, as was a sputum acid-fast bacilli (AFB) smear. On the second hospital day, his general condition and hypoxia deteriorated on a reserve mask with an oxygen flow rate of 15 L/min. He was transferred to intensive care unit and placed on mechanical ventilation. Flexible bronchoscopy for microbial evaluation showed no apparent endobronchial lesions, although bloody secretions were noted in the left upper and lower lobe bronchi. Bronchoalveolar lavage (BAL) performed in the lingular division of the left upper lobe revealed a white blood cell count of 2,480/mm3 (segmented neutrophils 18%, lymphocytes 52%, other cells 30%); red blood cell count of 100,000/mm3; no bacteria, viruses, or fungi; and negative AFB smear and culture results. All virus PCRs and microbial tests, including blood and urine cultures, were negative.
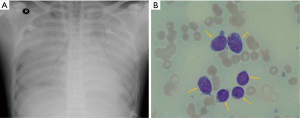
On the third hospital day, the bone marrow was biopsied after finding abnormal immature blast cells in a peripheral blood smear. Despite maximal ventilator support with inhaled nitric oxide therapy with prone position, his hemodynamic and ventilator parameters were deteriorated further and chest radiography rapidly worsened (Figure 2A). The PaO2/FiO2 ratio was 56, with a FiO2 of 1.0 and a positive end-expiratory pressure (PEEP) of 10 cm H2O. The Murray score was 3 and the Respiratory Extracorporeal Membrane Oxygenation Survival Prediction score was 4.
ECMO (Maquet Rotaflow Centrifugal Pumps with Quadrox-D oxygenators, Maquet, Rastatt, Germany) treatment was promptly established using the veno-venous method via the femorojugular route using biocoated circuits. The circuit had double access through a 17-Fr jugular catheter for oxygenated blood and a 19-Fr femoral catheter (Bio-medicusTM Medtronic, Minneapolis, MN) for deoxygenated blood to the pump. The gas exchange flow, initial oxygen fraction, pump speed, and cardiac index of VV ECMO were 6 L/min, 100%, 5,000 LPM and 2.8 L/min/m2, respectively. Nafamostat mesylate was used as an alternative to heparin at a dosage of 0.5–1 mg/kg/h to maintain an activated partial PT of 60–80 seconds. During ECMO support, the patient was sedated and mechanically ventilated using lung protective settings of FiO2 0.5, tidal volume 100–150 mL, a respiratory rate 10 breaths/min, and PEEP 10 cm H2O.
Acyclovir as a broad antiviral agent and Bactrim to cover Pneumocystis jiroveci were added intravenously. On the fifth hospital day, a bone marrow aspiration smear revealed that blasts made up to 64% of absolute neutrophil count (Figure 2B) and a leukemia marker study was positive for cMPO, CD34, CD117, CD13, CD33, CD38, CD123, HLA-DR. Consequently, AML (M1) was diagnosed and induction chemotherapy was started after consulting a hematologist, including 3 days with intravenous idarubicin 12 mg/m2, 7 days of intravenous cytarabine 200 mg/m2 and dexamethasone 40 mg/day. The antibiotic treatment was changed to intravenous vancomycin and meropenem to cover methicillin-resistant Streptococcus aureus and extended-spectrum beta-lactamase-positive bacteria and an intravenous antifungal agent was initiated.
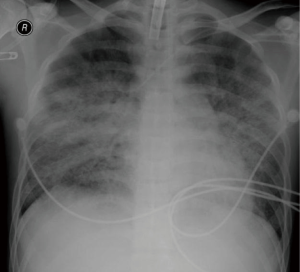
A bedside tracheostomy was performed on his 11th hospital day because of prolonged intubation. There were no signs of significant bleeding such as intracranial hemorrhage or secondary infection. On the 13th day, chest radiography showed improvements in his pneumonia and ARDS (Figure 3). As his vital signs were stable, the ECMO support was discontinued and he was maintained on a mechanical ventilator. His vital signs and PaO2 remained stable and he had no dyspnea on the day of weaning from ECMO. Our patient had been isolated in a clean room with limited access, and laboratory tests were performed every day to check for new infections or organ failure. He was weaned from mechanical ventilator on the 19th hospital day and transferred to general ward. Despite all of these efforts and weaning from ECMO and mechanical ventilator, he died during the consolidation chemotherapy as a result of septic shock with pancytopenia.
Discussion
ARDS may develop in patients with hematological malignancies, resulting in high morbidity and mortality. Chemotherapy often induces prolonged periods of marrow aplasia, leading to neutropenia, thrombocytopenia and defective immunity. Consequently, patients are more susceptible to bacterial, viral and fungal infections.
In patients with acute leukemia, pulmonary leukemic cell infiltration and subsequent pulmonary failure remain an unresolved problem and major cause of death (1). Immediately or shortly after induction chemotherapy, acute lysis pneumopathy can cause diffuse alveolar damage (9). Moreau et al. was reported that more than 80% of patients experienced acute respiratory events early days of the AML diagnosis and 61% of all respiratory events were related to leukemia-specific lung involvement (10). Therefore, there was probability of acute respiratory events related to AML such as pulmonary leukemic infiltration in our patient because we did not find any pathogen in our patient.
One technique to improve the survival of patients with hematological malignancies with pulmonary failure is ECMO, which markedly benefits critically ill patients with severe respiratory failure refractory to conventional treatment including mechanical ventilation (11). However, compared with standard indications for ECMO, chemotherapy-induced cytopenia and respiratory failure pose additional medical challenges for the treatment of patients with acute leukemia when considering ECMO. Patients with hematological malignancies and a suppressed immune system on ECMO can develop severe complications, such as infections and bleeding.
The risk of ECMO-induced septic episodes is increased in immunocompromised, severely neutropenic patients due to hematologic malignancies with chemotherapy. Even in immunocompetent patients, ECMO support is a high-risk procedure for nosocomial infection. Moreover, there is a high risk of bleeding and a markedly increased demand for transfusions during ECMO in acute leukemia and the need for anticoagulation can lead to hemorrhage with increased requirement for blood transfusions. These conditions remain a major cause of therapy-associated morbidity and death.
With improvements in ECMO technology and expertise lowering morbidity and mortality, the criteria for the use of ECMO may be extended to ARDS patients with hematological malignancies such as acute leukemia and lymphoma (7,12). Wohlfarth et al. (7) reported a remarkable 50% survival rate, despite the presence of numerous factors known to be associated with an adverse outcome in patients with hematological malignancies undergoing ECMO.
Consequently, we used veno-venous ECMO in this case. Our patient was a healthy young adult before this event, but he deteriorated rapidly at the initiation of chemotherapy. He was eligible candidate for ECMO as a rescue therapy because of very severe refractory hypoxemia with short high FiO2 ventilation period. ECMO was instituted urgently to restore hemodynamic stability, improve end-organ function, and bridge the patient during consequences of additional chemotherapy administration until the tumor burden could be adequately reduced. After completing induction chemotherapy, the ECMO was discontinued and the patient decannulated at the bedside.
There are some reports on weaning adult patients with hematological malignancies such as acute lymphoid leukemia, lymphoma, and multiple myeloma from ECMO (7,12). However, there are few reports of performing this during induction chemotherapy in an adult with AML (8). Our experience indicates that ARDS caused by pneumonia in patients with undergoing chemotherapy for acute leukemia should not be considered a contraindication to ECMO treatment. As successful treatment is associated with early initiation, we suggest that ECMO be started in the presence of severe hemodynamic instability or respiratory failure refractory to standard medical support.
In conclusion, ECMO is a supportive tool that reduces the incidence of early treatment-related mortality and will ultimately improve the overall survival in patients with ARDS and pneumonia during induction chemotherapy for acute leukemia.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Azoulay E, Mokart D, Pène F, et al. Outcomes of critically ill patients with hematologic malignancies: prospective multicenter data from France and Belgium-a groupe de recherche respiratoire en réanimation onco-hématologique study. J Clin Oncol 2013;31:2810-8. [Crossref] [PubMed]
- Vadde R, Pastores SM. Management of Acute Respiratory Failure in Patients with Hematological Malignancy. J Intensive Care Med 2015. [Epub ahead of print]. [PubMed]
- Azoulay E, Mokart D, Lambert J, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med 2010;182:1038-46. [Crossref] [PubMed]
- Kasirajan V, Smedira NG, McCarthy JF, et al. Risk factors for intracranial hemorrhage in adults on extracorporeal membrane oxygenation. Eur J Cardiothorac Surg 1999;15:508-14. [Crossref] [PubMed]
- Maclaren G, Butt W. Extracorporeal membrane oxygenation and sepsis. Crit Care Resusc 2007;9:76-80. [PubMed]
- Worku B, DeBois W, Sobol I, et al. Extracorporeal Membrane Oxygenation as a Bridge through Chemotherapy in B-Cell Lymphoma. J Extra Corpor Technol 2015;47:52-4. [PubMed]
- Wohlfarth P, Ullrich R, Staudinger T, et al. Extracorporeal membrane oxygenation in adult patients with hematologic malignancies and severe acute respiratory failure. Crit Care 2014;18:R20. [Crossref] [PubMed]
- Kang HS, Rhee CK, Lee HY, et al. Clinical outcomes of extracorporeal membrane oxygenation support in patients with hematologic malignancies. Korean J Intern Med 2015;30:478-88. [Crossref] [PubMed]
- Tryka AF, Godleski JJ, Fanta CH. Leukemic cell lysis pneumonopathy. A complication of treated myeloblastic leukemia. Cancer 1982;50:2763-70. [Crossref] [PubMed]
- Moreau AS, Lengline E, Seguin A, et al. Respiratory events at the earliest phase of acute myeloid leukemia. Leuk Lymphoma 2014;55:2556-63. [Crossref] [PubMed]
- Ventetuolo CE, Muratore CS. Extracorporeal life support in critically ill adults. Am J Respir Crit Care Med 2014;190:497-508. [Crossref] [PubMed]
- Gorjup V, Fister M, Noc M, et al. Treatment of sepsis and ARDS with extracorporeal membrane oxygenation and interventional lung assist membrane ventilator in a patient with acute lymphoblastic leukemia. Respir Care 2012;57:1178-81. [Crossref] [PubMed]


