Surgery and perioperative management for post-intubation tracheoesophageal fistula: case series analysis
Introduction
Post-intubation tracheoesophageal fistula (PITEF) is a rare complication of prolonged mechanical ventilation (1,2). Variable, but generally severe clinical issues are usually associated with this condition including malnutrition, recurrent pneumonia, infections, frequent neurological disorders and/or ventilator dependence. Furthermore, surgery is challenging due to a variety of technical issues; especially tissue damage and local infection (3,4). Since pre-operative preparation, appropriate general supportive therapy and surgery timing and technique can influence patient outcome, we reviewed for both the choice and timing of surgical technique and outcome in PITEF patients.
Methods
We reviewed all past cases of PITEF between 2000 and 2014 that underwent the Grillo surgical method. For all included cases, tracheoesophageal fistulas secondary to other causes were excluded, and only those cases treated with primary repair of the esophagus and airway resection/reconstruction were included.
We set up a database to collect the following information: cause and duration of intubation, observed comorbidities, nutritional and infective statuses, weaning time from mechanical ventilation, diagnostic work up, fistula size, fistula location, fistula distance from the glottic plane, previous tracheostomy, any subglottic larynx injury, condition of whole trachea, preparation for surgery, selected operative technique, postoperative course, treatment of surgical complications and long term outcome. The time spans from the last days of mechanical ventilation were also recorded. All 9/10 surviving patients at five year follow-up underwent complete clinical examination and bronchoscopy. As this was a case-series review of few patients, no statistical analysis was appropriate. This review was carried out with the approval from both the Perugia and Terni University Hospitals Institutional Review Boards.
Results
Population overview
Overall, 104 patients had undergone tracheal resection and reconstruction for different indications. Among these, 10 patients were submitted for primary esophageal suture and tracheal resection-reconstruction for PITEF. In this subgroup we included patients who had been mechanically ventilated for long-lasting coma (9 neurologic, 1 metabolic). There were 6 females and 4 males, median age 39 years (range, 26–77 years). The clinical characteristics at the time of referral are summarized in Table 1.
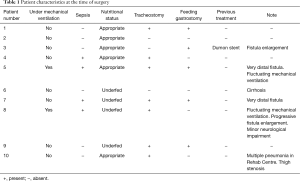
Full table
Preparation for surgery and preoperative assessment
Four patients were in good condition and did not need preoperative preparation (#1, #2, #3, #10). Whereas, for the remaining six patients, surgical treatment was followed by a period of antibacterial therapy, enteral feeding and/or permanent weaning from a ventilator.
Patients were evaluated according to general conditions: debilitated when BMI <20, severe hypoproteinemia, electrolyte imbalance, renal imbalance, cardiorespiratory failure or coma, as suggested by a current clinical practice recommendation (5). Infection-related illness was deemed in the case of septic fever and/or pneumonia in the previous month, associated to significant elevation of erythrocyte sedimentation rate and C-reactive protein. All the available clinical data were evaluated as prognostic factors and decision-making process indicators. We utilized the P-Possum score to estimate the level of impairment (Table 2).
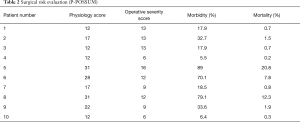
Full table
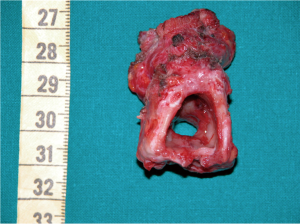
The preparation for curative surgery included percutaneous gastrostomy tube placement and tracheostomy in 3/10 patients (#5, #7, #9). Another patient had been treated two years earlier, at another hospital, with gastrostomy and tracheal stenting (Dumon stent 14–40 mm) (#3); the original endoscopic report, before stent placement, described a 4mm fistula (Figures 1,2). Two patients (#5, #8) underwent surgery, despite the unsatisfactory results from long term preparation, for different reasons: (I) enlarging fistula and multiple recurrent pneumonia with repeated restart of a previously discontinued mechanical ventilation (#8); (II) distal PITEF with difficult placement of the tube cuff below the fistula; this young patient was difficult to wean from the ventilator for neurological reasons (#5). Both patients had suitable anatomies for performing an ulterior tracheostomy below the anastomosis.
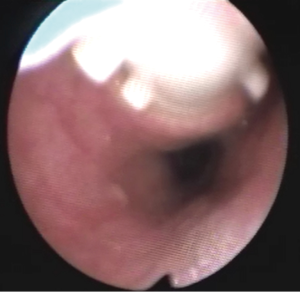
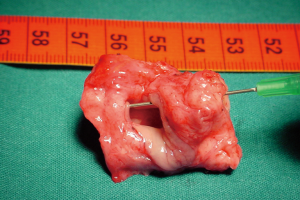
A preliminary rigid bronchoscopy was performed in all cases to assess the condition of the trachea, the length of PITEF and its exact position. The mean longitudinal length of the fistula was 20.5 mm (median 17.5 mm; range, 8–45 mm); the median distance between the glottic plane and the distal limit of PITEF was 43 mm (range, 20–68 mm). A concomitant tracheal stenosis was diagnosed in 7/10 patients: the fistula was located immediately below the stenosis in 3 patients (Figure 3), and much more distally in 4. A median 28 mm length (range, ≅0–40 mm) was recorded regarding the segment of airway removed.
Surgical technique
All the surgical procedures were performed in a single stage with a nasogastric tube positioned. Despite the presence of tracheostomy, all patients were intubated, facilitated by a flexible bronchoscope, carefully placing the orotracheal tube below the fistula. Surgery was performed through a collar incision and a sternal split was added in two cases: one of these was for the patient who underwent second surgery.
Even though all patients underwent a transtracheal suture of the fistula, data analysis highlighted significant technical variations; likely influenced by anatomical features. Most patients (#1, #2, #3, #4, #6, #10) underwent resection of the airway tract including the fistula site. Here, the trachea was circumferentially dissected just below the PITEF and transected at this level. Cross-field ventilation was then started by intubation of the distal tracheal stump. The fistula was excised starting from the bottom, then proceeding upward to the proximal tracheal stump. Therein, the esophagus was dissected just enough to expose the fistula. When the fistula was completely divided, the esophageal mucosa was repaired with longitudinal interrupted inverting sutures (4/0 Vicryl, Ethicon Inc., Cornelia, GA, USA) and the esophageal muscular layers were closed using interrupted everting sutures. A caudally pedicled sternohyoid muscle flap was interposed between the esophagus and airway, and thereafter, secured with multiple stitches to the muscle layer of the esophagus, in order to completely cover the sutured fistula. The proximal tracheal stump was further dissected until reaching undamaged mucosa and then transected at this level. Finally, tracheal anastomosis was completed with interrupted sutures (2/0 and 4/0 Vicryl) according to a description by Mathisen et al. (6).
In 3/10 cases, the fistula was located very distally to the damaged trachea, and for this, resection of the entire tract inclusive of the fistula and tracheal stenosis was not deemed feasible (patients #5, #7, #8). These patients underwent tracheal or laryngotracheal (#7, #8) resection, as well as reconstruction, without excision of the fistula site. The airway was cut above the fistula and the trachea was separated from the esophageal wall. The fistula was then divided, preserving the mucosa for reconstruction of the trachea rather than the esophagus (7). After the trachea was completely freed, the defect in the esophagus was repaired and the pedicled muscle flap was secured over the esophageal suture. The posterior defect of the distal tracheal stump at the level of the excised fistula was also repaired, by suturing the posterior membranous wall with 4/0 Vicryl interrupted everting stitches. Finally, resection and reconstruction of the proximal, damaged trachea was performed; resulting in a shortened tracheal segment.
In one patient (#9), the airway injury was considered too long to permit a safe tracheal reanastomosis. So, the procedure consisted of an esophageal suturing through a transtracheal approach, followed by a short tracheal resection including only the malacic segment at the level of the original tracheostomy, then a tracheal anastomosis was performed, and finally a second tracheostomy was performed at the level of the anastomosis, followed by a permanent T-Tube placement (Hood Laboratories, Pembroke, MA USA). In this case, the T-Tube was successfully removed at 35 months. A small tracheostomy was performed in two other patients (#5, #8) consisting of placing the device a couple of rings below the tracheal anastomosis. This was done to allow for mechanical ventilation, if needed.
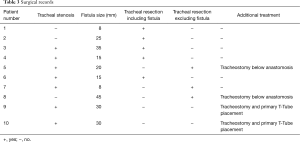
An anterior flexion of the head was maintained for seven days using the chin-sternal stitch. All recorded surgical treatment data are reported in Table 3.
Full table
Data analysis
One postoperative fatal esophageal bleeding, due to liver cirrhosis, was registered on day 9 (#6). This patient had also shown an anastomotic complication on day 5 and never restarted oral feeding postoperatively. Two other cases of tracheal suture leakage occurred. One of these was a partial dehiscence, with granulomas formation at the anastomotic site without fistula reopening, which developed in a temporarily ventilator-dependent patient (#8). In this case, the tracheostomy below the anastomosis had already been performed and a size 12 Montgomery T-Tube was placed, because of spontaneous breathing: oral intake was restarted on day 20 and the T-Tube was left in place for 11 months, resulting in mild, asymptomatic tracheal stenosis following the tracheostomy closure. The second patient had a reopening of the fistula (#7). Being so, a tracheostomy was performed at the site of the previous anastomosis. Forty days later, the same patient underwent a redo cervicotomy including a sternal split and following this the fistula was closed using a transtracheal suture and a T-Tube was positioned. This procedure allowed for the permanent closure of the fistula, but the tracheostomy was permanent and only after 50 days was the T-Tube substituted with a tracheostomy tube, due to impaired neurological conditions. The remaining seven patients had a “uneventful recoveries” and the median post-operative hospital stay was 11 days.
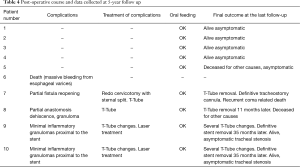
Overall, 7/10 patients had a follow up at 5 years from surgery consisting of a thorough clinical examination to assess for normal breathing parameters feeding. Two patients had died before follow up and family members reported that the patient deaths were associated to cardiovascular disease and not to PITEF. All nine interviews with family members led to claims that all the patients had maintained oral feeding following hospital discharge. At the 5-year follow up, normal range breathing was observed in the 7 patients (1 with the T-Tube) while in 1 of these patients, breathing was feasible only with a permanently positioned tracheostomy tube. Both post-operative course and information collected at 5-year follow up are summarized in Table 4.
Full table
Discussion
PITEF are triggered by chronic pressure, which in turn leads to ischemia: an endotracheal tube cuff hypertension that presses on the nasogastric tube (8,9). These patients are often debilitated or mechanically ventilated and frequently require to palliative procedures. Currently, surgery is the only curative treatment, given that spontaneous healing is extremely unlikely and it is only possible in the acute setting. Repair should be anticipated with several essential preliminary steps including both weaning from mechanical ventilation and resolution of any other medical impairment. If discontinuation of mechanical ventilation is impossible, most authors suggest promptly adopting palliative initiatives to prevent complications such as nasogastric tube removal, cuff placement distal to the fistula, drainage gastrostomy and feeding enterostomy. Thus, an enlargement of the fistula and inhalation of any gastroesophageal reflux can be avoided, while mechanical ventilation and proper nutrition are allowed (10-12). However, some authors have described these disadvantages from delayed treatment (13).
Therefore, the choice of surgical technique is still a matter of debate (14-20). That is, PITEF repair can be carried out by direct suturing through a transtracheal approach, or through a lateral exposure of the fistula, without tracheal division. In both approaches, there is unanimous agreement, that the suturing of the fistula should be protected by an interposed muscle flap. In fact, a left sternohyoid muscle flap, caudally pedicled, was tailored to provide the most effective protection (18).
Regarding esophageal diversion, it can be associated with a high complication rate and therefore strict patient selection is recommended (12).
We have no experience with the lateral approach to the fistula, which is generally considered as a technique with narrow indications, basically featured by very small defects, without tracheal wall damage. This technique basically leads to an inadequate surgical access to the fistulous tract and can be also followed by several complications as laryngeal nerve injury, fistula recurrence, late tracheal stenosis in the suture site (10,12,17,18). In 1976, Grillo et al. (10) suggested the direct suturing of the esophageal site and the resectioning of the affected trachea through an anterior cervicotomy with or without sternal splitting. Several years later, Mathisen et al. (6) validated this approach, which today is considered the treatment gold standard for PITEF. The advantages of the Grillo technique include an excellent visual access to the fistula and the lowest risk of tracheal devascularization. Another benefit of the transtracheal approach over the lateral one is that it carries a lower risk of laryngeal nerve injury; which occurred only in the patient submitted to redo surgery for a giant PITEF proximal to the cricoid. Concomitant tracheal stenosis is common in PITEF. However, any decision regarding tracheal resection needs to take into consideration any pre-existing tracheal injuries. The surgical technique for PITEF allows for a direct access to the fistula, which is ensured by a division of the trachea. Whereas, in the case of an undamaged airway, the shortest tracheal resection has not been reported to add morbidity.
The closure of the fistula is the aim of the treatment. However, surgery for PITEF is challenging and its related morbidity rate is not negligible; certainly greater than tracheal surgery for malignancy (21,22). In fact, the complication rate has been reported to be between 32% and 56%, mainly anastomotic issues or fistula recurrences (3,4,23). The results from our case series review regarding complications are in line with published literature. Specifically, patients with PITEF often have major comorbidities and are also debilitated, despite a preoperative preparation. Furthermore, the trachea and esophagus are always stuck together and therein a circumferential dissection of the trachea below the fistula is technically demanding. Likewise, a resectioning of a long tracheal segment is sometimes required for tracheal injury distant from the fistula, and for a possible concomitant tracheostomy. Conversely, when a large fistula is located immediately below the cricoid, is challenging to detach residual esophageal mucosa from the cricoid cartilage.
In our experience, PITEF presents significant features of inter-individual variability and the lesion can differ in terms of size, position, deterioration and symptoms. Local assessment of operability is mainly based on the endoscopic evaluation. Rigid bronchoscopy is still an optimal method to get a clear picture of the local condition in order to plan and prepare the most appropriate surgical procedure (24).
When the segment of trachea to resect is too long, other technical solutions must include the primary aim of fistula closure as well as the repairing of the tracheal stenosis as a secondary aim. A pre-existing tracheostomy stoma and the possibility of placing a new tracheotomy below the tracheal anastomosis are considered non-negligible parameters. Resections of segments including the fistula, tracheostomy and stenosis can be risky. Here, there are two surgical options: (I) PITEF closure, ensuring the tracheal patency utilizing permanent stenting with a T-Tube; (II) esophageal suturing, repair of the tracheal membranous wall adjacent to the fistula and a resectioning limited to the cranially-located stenotic segment. Using this approach, a significant reduction in the length of the trachea to resect can be achieved. After the esophageal repair has been completed, the membranous tracheal wall could be directly sutured whenever it is not severely damaged. In such circumstances, the fistula needs be incised sparing the tissues in favour of the trachea, in order to avoid a postoperative tracheal stenosis. Actually, any moderate loss of esophageal mucosa is usually well tolerated.
Surgery must be considered very cautiously in patients dependent on mechanical ventilation (25), even though, in our opinion, ventilator dependence should not be considered an absolute contraindication to the surgical closure of the fistula. Every single case must be assessed individually. Specifically, although complete weaning from mechanical ventilation is preferred, some patients are ventilator dependent because an infection secondary to repeated airway soiling and this does not allow for weaning.
The non-operative options available for PITEF treatment include bronchocatheters, long straight or bifurcated tracheostomy tubes; both should be considered for inoperable patients (26). In the ten reviewed cases of this case series, the surgical indication was also extended to two patients who were not completely weaned from the ventilator: the prerequisite was the feasibility of a small tracheostomy a couple of rings below the anastomosis. Placing a tracheostomy below the suture line is sometimes possible after tracheal resection for PITEF. Indeed, if a suitable anatomy is deemed in a patient with repeated pulmonary infections and/or progressive enlargement of the fistula, an aggressive surgical treatment can be justified. Good post-operative outcomes were achieved, despite the need for a short postoperative period of mechanical ventilation, in the two patients who could not be completely weaned from ventilators before surgery. In fact, a complete weaning from mechanical ventilation in these two patients was achieved only when the fistula had been permanently closed; even though one of these patients developed an anastomotic complication, which was successfully treated with a temporary T-Tube.
The T-Tube is an essential device in tracheal surgery but its role in the treatment of PITEF is less clear. It can be used to treat anastomotic complications in PITEF patients, or when tracheal damage is excessive and therefore prevents an entire resectioning of the diseased airway. Nonetheless, surgeons need to carry out the primary objective of this procedure, which is to close the fistula. In fact, permanent PITEF closure and T-Tube placement are associated with good outcome, given the fact that T-Tube removal can occur even months after stenting for airway lumen stabilization (27). Furthermore, a modified T-Tube with the cranial branch occluded by an internal inflatable balloon, was successfully used for temporary mechanical ventilatory support in selected patients, treated with direct repair of the PITEF and tracheal or laryngotracheal resection (28). Likewise, a well-positioned T-Tube does not exert any radial tension on the tracheal wall and keeps its position because the horizontal branch is bound to the tracheostomy. For this, it is not believed to cause ischemia of the adjacent mucosa and does not seem to interfere with the healing of a recently performed tracheal anastomosis. To this regard, one of the cases in this review was deemed unresectable due to extended airway damage and the digestive fistula closure was performed along with a limited tracheal resection which included the placement of a trans-anastomotic T-Tube, which was intended to be permanent. Despite the pessimistic outlook immediately following surgery, an adequate airway lumen was obtained and permanent decannulation with the tracheostomy closure was achieved months after stenting with a T-Tube. Indeed, authors have reported good benefit from tracheobronchial stenting for the conservative treatment of PITEF in patients with a clinically assessed poor prognosis including the need for mechanical ventilation (29). Given that fistulas can be caused or worsened by self-expandable stents, their use in treating PITEF might appear to be a contradictory solution (30). However, one of the patients in our case series had had a tracheal Dumon stent and gastrostomy for some years. Despite this, the stent allowed for normal ventilation which in turn may have avoided recurrent episodes of pneumonia, but resulted in a three-fold enlargement of the fistula, which included the entire width of the membranous tracheal wall, at the time of surgery (Figure 2).
In conclusion, this review was of a single Institution with limitations inherent of a retrospective study. This review is without a comparison analysis, since only one surgical technique was performed. Our review results suggest that primary esophageal closure with tracheal resection/reconstruction is an effective treatment for most PITEF patients; although associated morbidities are not uncommon. This conclusion is in agreement with a large number of past retrospective studies (3,4,6,7,14,23). Moreover, this case series review suggests that even ventilator-dependent patients may benefit from surgery when they are selected according to a criterion based upon general condition, worsening trend and fistula size. Finally, the T-tube assisted in stabilizing complicated cases and often resulted being a permanent solution.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: All patients gave informed consent. The Institution (University Hospitals of Perugia and Terni) approved this series analysis.
References
- Harley HR. Ulcerative tracheo-oesophageal fistula during treatment by tracheostomy and intermittent positive pressure ventilation. Thorax 1972;27:338-52. [Crossref] [PubMed]
- Flege JB Jr. Tracheoesophageal fistula caused by cuffed tracheostomy tube. Ann Surg 1967;166:153-6. [Crossref] [PubMed]
- Muniappan A, Wain JC, Wright CD, et al. Surgical treatment of nonmalignant tracheoesophageal fistula: a thirty-five year experience. Ann Thorac Surg 2013;95:1141-6. [Crossref] [PubMed]
- Marulli G, Loizzi M, Cardillo G, et al. Early and late outcome after surgical treatment of acquired non-malignant tracheo-oesophageal fistulae. Eur J Cardiothorac Surg 2013;43:e155-61. [Crossref] [PubMed]
- Campillo B, Paillaud E, Uzan I, et al. Value of body mass index in the detection of severe malnutrition: influence of the pathology and changes in anthropometric parameters. Clin Nutr 2004;23:551-9. [Crossref] [PubMed]
- Mathisen DJ, Grillo HC, Wain JC, et al. Management of acquired nonmalignant tracheoesophageal fistula. Ann Thorac Surg 1991;52:759-65. [Crossref] [PubMed]
- Grillo HC. Repair of acquired tracheoesophageal and bronchoesophageal fistula. In: Grillo HC. editor. The Surgery of Trachea and Bronchi. Toronto: BC Decker Inc., 2004:572-4.
- Payne DK, Anderson WM, Romero MD, et al. Tracheoesophageal fistula formation in intubated patients. Risk factors and treatment with high-frequency jet ventilation. Chest 1990;98:161-4. [Crossref] [PubMed]
- Seegobin RD, van Hasselt GL. Endotracheal cuff pressure and tracheal mucosal blood flow: endoscopic study of effects of four large volume cuffs. Br Med J (Clin Res Ed) 1984;288:965-8. [Crossref] [PubMed]
- Grillo HC, Moncure AC, McEnany MT. Repair of inflammatory tracheoesophageal fistula. Ann Thorac Surg 1976;22:112-9. [Crossref] [PubMed]
- Macchiarini P, Verhoye JP, Chapelier A, et al. Evaluation and outcome of different surgical techniques for postintubation tracheoesophageal fistulas. J Thorac Cardiovasc Surg 2000;119:268-76. [Crossref] [PubMed]
- Urschel HC Jr, Razzuk MA, Wood RE, et al. Improved management of esophageal perforation: exclusion and diversion in continuity. Ann Surg 1974;179:587-91. [Crossref] [PubMed]
- Thomas AN. Management of tracheoesophageal fistula caused by cuffed tracheal tubes. Am J Surg 1972;124:181-9. [Crossref] [PubMed]
- Gerzić Z, Rakić S, Randjelović T. Acquired benign esophagorespiratory fistula: report of 16 consecutive cases. Ann Thorac Surg 1990;50:724-7. [Crossref] [PubMed]
- Fiala P, Cernohorský S, Cermák J, et al. Tracheal stenosis complicated with tracheoesophageal fistula. Eur J Cardiothorac Surg 2004;25:127-30. [Crossref] [PubMed]
- Le Brigand H, Roy B. Tracheo-oesophageal fistulas after tracheotomy. Apropos of 4 cases. Mem Acad Chir (Paris) 1966;92:405-16. [PubMed]
- Couraud L, Bercovici D, Zanotti L, et al. Treatment of esophagotracheal fistula following intensive care. An experience of 17 cases. Ann Chir 1989;43:677-81. [PubMed]
- Santini P, Dragotto A, Gigli PM, et al. Postintubation tracheoesophageal fistula: surgical treatment of three cases. J Thorac Cardiovasc Surg 1998;116:518-9. [Crossref] [PubMed]
- Baisi A, Bonavina L, Narne S, et al. Benign tracheoesophageal fistula: results of surgical therapy. Dis Esophagus 1999;12:209-11. [Crossref] [PubMed]
- Camargo JJ, Machuca TN, Camargo SM, et al. Surgical treatment of benign tracheo-oesophageal fistulas with tracheal resection and oesophageal primary closure: is the muscle flap really necessary? Eur J Cardiothorac Surg 2010;37:576-80. [Crossref] [PubMed]
- Avenia N, Vannucci J, Monacelli M, et al. Thyroid cancer invading the airway: diagnosis and management. Int J Surg 2016;28 Suppl 1:S75-8. [Crossref] [PubMed]
- Andolfi M, Vaccarili M, Crisci R, et al. Management of tracheal chondrosarcoma almost completely obstructing the airway: a case report. J Cardiothorac Surg 2016;11:101. [Crossref] [PubMed]
- Bibas BJ, Guerreiro Cardoso PF, Minamoto H, et al. Surgical Management of Benign Acquired Tracheoesophageal Fistulas: A Ten-Year Experience. Ann Thorac Surg 2016;102:1081-7. [Crossref] [PubMed]
- Daddi G, Puma F, Avenia N, et al. Resection with curative intent after endoscopic treatment of airway obstruction. Ann Thorac Surg 1998;65:203-7. [Crossref] [PubMed]
- Goldstone J. The pulmonary physician in critical care. 10: difficult weaning. Thorax 2002;57:986-91. [Crossref] [PubMed]
- Tazi-Mezalek R, Musani AI, Laroumagne S, et al. Airway stenting in the management of iatrogenic tracheal injuries: 10-Year experience. Respirology 2016;21:1452-8. [Crossref] [PubMed]
- Puma F, Ragusa M, Avenia N, et al. The role of silicone stents in the treatment of cicatricial tracheal stenoses. J Thorac Cardiovasc Surg 2000;120:1064-9. [Crossref] [PubMed]
- Daddi N, Tassi V, Belloni GP, et al. The surgical repair of benign tracheo-oesophageal/pharyngeal fistula in patients on mechanical ventilation for severe neurological injuries†. Eur J Cardiothorac Surg 2016;49:1279-81. [Crossref] [PubMed]
- Lin WY, Chiu YC. Complete healing of tracheoesophageal fistula in a ventilator-dependent patient by conservative treatment. Respirol Case Rep 2014;2:27-9. [Crossref] [PubMed]
- Han Y, Liu K, Li X, et al. Repair of massive stent-induced tracheoesophageal fistula. J Thorac Cardiovasc Surg 2009;137:813-7. [Crossref] [PubMed]


