Mediastinal transposition of the omentum reduces infection severity and pharmacy cost for patients undergoing esophagectomy
Introduction
According to a 2012 World Health Organization report, esophageal cancer is the eighth most prevalent cancer worldwide, with an estimated 456,000 new cases and 400,000 causal deaths within the same year (1). Although endoscopic intervention is effective in stage I esophageal cancer, surgery remains the primary treatment modality for locally advanced esophageal cancer. However, a preoperative malnutrition state, extensive surgery, and technical complexity all contribute to the high morbidity and reduce the survival rate of surgical patients after esophagectomy (2).
The clinical application of esophagectomy followed by esophagogastric anastomosis is prone to anastomotic leaks-a common but devastating postoperative complication predisposed by several intrinsic anatomical factors specific to the esophagus, such as lack of serosa, a longitudinal muscle layer, and technical difficulty (3). Historically, postoperative leaks have been related to high mortality (above 60%) with thoracic anastomosis (4,5), regardless of various attempts made to counteract the disseminating inflammation involved in this process. Over time, novel techniques such as mechanic stapling and omentum buttressing (6) have been combined with enhanced anastomosis to prevent leakage. Aggressive enteral nutrition support, timely intervention, and the improved experiences and resources available at high-volume centers have drastically reduced leak-associated mortality (7).
The greater omentum was originally found to adhere to gastrointestinal perforations during early abdominal surgery. It was later identified as an immune organ, which inspired its use to reinforce anastomosis in surgeries. In relation to esophagectomy, several studies on omentoplasty involving the intuitive wrapping of an omental graft around an anastomotic site, have suggested a protective effect as seen in colorectal surgery (6,8-13). As anastomotic leaks become less associated with increased mortality (7), it is reasonable to predict a shift in the etiology of postoperative mortality from a solitary leak to a broader spectrum with a distinct proportion of intrathoracic infection. In that case, the conventional omental wrapping would be questionable as an optimal approach to using the omentum for prophylactic purposes. Thus, we thought to modify the technique by transposing the omentum to the post-tracheal mediastinal space instead of sealing the anastomosis circumferentially. In such circumstances, the omentum has more mobility and is capable of encompassing overall postoperative intrathoracic complications, including infection. This study was performed to investigate the efficacy and cost-effectiveness of the omentum mediastinal transposition technique.
Methods
Patients
Two hundred eighteen consecutive patients diagnosed with esophageal cancer were treated with a curative Ivor-Lewis esophagectomy via open approach at the First Affiliated Hospital of Zhejiang University from January 2010 to March 2015. All of the patients underwent a thorough examination before surgery, and endoscopic biopsies were performed to confirm malignancy. Integrated positron emission tomography/computed tomography (PET-CT) was used to assess patients suspected of distant metastasis. Neoadjuvant chemotherapy was given to select patients with large tumors to confer operability for radical resection. Pathologic staging was determined according to the seventh edition of the AJCC Cancer Staging Manual for esophagus and esophagogastric junction cancers (14). The entry criteria were (I) no previous history of esophageal or gastric surgery; (II) no history of other malignancies, and (III) at least six resected lymph nodes (15). Two patients were excluded due to inadequate lymph node resection and eight were excluded due to a history of other malignant diseases. Two hundred eight patients were ultimately enrolled in the study. The study received approval from the Ethics Committee of The First Affiliated Hospital, School of Medicine, Zhejiang University (No. 2014346). A waiver of consent was granted for this retrospective data review.
Surgery
All of the patients underwent an open Ivor-Lewis esophagectomy and esophagogastric anastomosis conducted by a single group of experienced surgeons under general anesthesia. During the operation, a gastric tube (4–5 cm wide) was crafted into a conduit by cutting 2 to 3 times along the lesser curvature with a linear stapler. A jejunostomy tube was routinely placed for postoperative enteral nutrition. Anastomosis was achieved with a 25-mm circular stapler using an intraluminal technique.
In 121 patients, an additional procedure was performed to transpose the greater omentum to the mediastinum. The decision to perform the procedure was made according to the operator’s discretion. During the procedure, the greater omentum was first mobilized by division along the greater curvature. The short gastric vessels were ligated, with 2 to 3branches starting from the proximal right gastroepiploic artery reserved as the only source of blood supply to the omentum flap. After mobilizing its distal attachment to the transverse colon, the pedicled omentum was fully prepared (Figure 1). It was then brought up into the thorax along with the gastric conduit to fill in the mediastinal space between the airway and the gastric conduit with the appropriate tension (Figure 2). Once the gastric conduit was in place, the omental flap underneath was compressed laterally to form a C-shape coating the conduit from a horizontal view. Three stitches were then placed to fix the artificial omental flap to the gastric conduit to prevent displacement due to body movement.
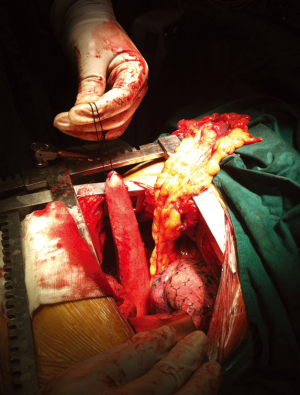
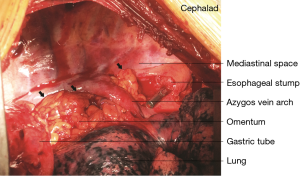
Postoperative care and monitoring
Postoperatively, the patients were monitored and cared for on a general thoracic ward unless otherwise indicated for intensive care unit transfer. Jejunostomy tube feeding was initiated between 24–48 h postoperatively. Oral feeding was allowed immediately after nasogastric tube removal on postoperative day 5–7. Routine chest radiography or computed tomography was scheduled after the first postoperative week to examine intrathoracic complications. Prophylactic antibiotics were routinely prescribed after surgery. In cases of infection, the empirical use of antibiotics was continued until evidence was obtained from culture and sensitivity testing. Whenever an anastomotic leak was clinically suspected, a methylene blue test, a gastrografin contrast study, and/or an upper gastrointestinal tract endoscopy were performed to evaluate conduit integrity.
Definition of intrathoracic infection
Any interpretation of infectious manifestation other than a leak within the thoracic cavity in postoperative radiographic studies was defined as intrathoracic infection, which frequently presented as pneumonia or empyema. With regard to disease severity, we further classified the patients into three categories i.e. intrathoracic infection free, covert intrathoracic infection, and overt intrathoracic infection (Table 1). The defining criteria were derived from a counterpart proposed at the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference (16). The management was based on clinical findings and the patients’ overall status.

Full table
Statistical analyses
To compare the associated variables between the omental transposition and non-transposition groups, the student t-test and Mann-Whitney U test were used for quantitative data while the Pearson χ2 and Fisher’s exact test were applied to qualitative data. Ranked intrathoracic infection data were analyzed via Kruskal-Wallis analysis and multivariate logistic regression analysis using a forward stepwise likelihood ratio test. The values of P<0.05 were taken to be statistically significant. All of the analyses were performed using the SPSS statistical software package 20.0.
Results
Demographics and pathologic characteristics
Table 2 presents the patients’ demographic and clinicopathologic characteristics with and without omental transposition. The population primarily comprised male patients (86.5%) with an average age of 63.2 years. Of 208 patients, 121 received omentum mediastinal transposition during the surgery. There were no significant differences with regard to the presence of smoking and alcohol history, common comorbidities or neoadjuvant therapy. The tumors were predominantly identified as squamous cell carcinomas (93.8%) under pathologic review, and primarily located in the middle thoracic esophageal region (69.2%). Pathologic stage 2 and 3 tumors constituted 38.9% and 47.1% of the population, respectively.
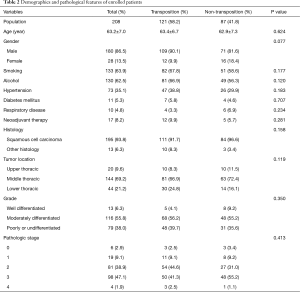
Full table
In-hospital postoperative complications
Radiologic screening revealed intrathoracic infections in 79 of the patients, as shown in Table 3. Of these, 37 cases belonged to the transposition group, which was lower in incidence than the non-transposition group for the other 42 cases (30.6% vs. 48.3%, P=0.009). At discharge, the patients who were supplemented with transposed omentum had a significantly shorter postoperative hospital stay than those without omentum, with a median postoperative stay of 14 vs. 16 days (P=0.038). The incidence of other major postoperative complications, including anastomotic leaks, was comparable between the two groups. Of note, the eight patients with leaks who had undergone omental transposition were all relieved over the treatment period, whereas the six patients in the non-transposition group with leaks were critically ill at presentation and progressed rapidly into septic shock, with two ultimately dying.
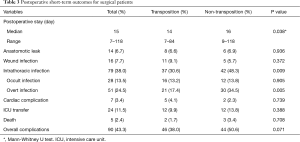
Full table
The patients were further classified by postoperative intrathoracic infection state, the criteria for which are presented in Table 1. The analysis revealed a statistically significant difference in the severity scale between the two groups of patients, with the non-transposition group proving more prone to significant intrathoracic infection (P=0.005), though a non-significant was found in overall complications (P=0.071), as displayed in Table 3.
Table 4 presents the multivariate logistic regression analyses for the risk factors associated with postoperative intrathoracic infection. The multivariate analysis identified omental transposition (OR=0.415, P=0.007) as an independent protective factor against postoperative intrathoracic infection.
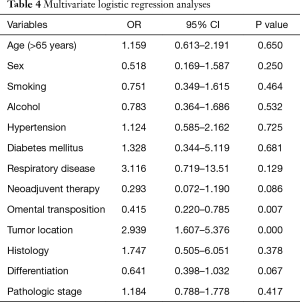
Full table
In-hospital treatment cost
Table 5 compares the in-hospital treatment costs (in Chinese yuan) for both groups. The median overall expense of each patient was similar in both groups (P=0.275). However, omental transposition was associated with lower pharmacy cost (P=0.010) but higher surgery cost (P=0.005).

Full table
Discussion
In early surgeries, the greater omentum was observed engaging in special movements toward the site of insult under a variety of clinical situations. Morrison linked it to some protective functions and recognized the omentum as the “abdominal policeman” (17). Later studies confirmed a reservoir of inflammatory cells within the omentum responsible for its immunological and tissue regenerative functions, which led to its use in complicated major surgeries to achieve better outcomes. Zhang et al. (9) reported the omentum reinforcement of esophagogastric anastomosis in 100 cases and no anastomotic leakage was observed postoperatively. The study did not set a control group, and the result was not analyzed statistically. However, three prospective randomized trials published more recently have strongly implicated the efficacy of omentoplasty in the prevention of anastomotic leaks. Bhat (11) and Dai (6) described the advantage of a lower incidence of anastomotic leakage under omental wrapping on postoperative outcomes after transhiatal and transthoracic esophagectomy. In another prospective study based on cervical anastomosis by Zheng (13), a similar result was observed within the omentoplasty group. Nevertheless, these studies have exclusively centered on the prevention of anastomotic leakage by wrapping the omentum around the anastomosis.
Given that intrathoracic leaks are less lethal (7) and pulmonary complications tend to increase after an esophagectomy (18), both conditions require equal attention in postoperative management. Likewise, the greater omentum is potentially motile in theory. On activation, it should spontaneously adhere to and defend the irritated area (17). This led us to wonder whether anastomosis wrapping had any alternative that might address the overall intrathoracic complications along with any anastomotic leaks. Thus, the idea of mediastinal transposition was born.
Of the 121 patients who underwent omental transposition, intrathoracic infection was present in 37 (30.6%)—significantly less frequent than in patients who underwent the pure Ivor-Lewis surgery (48.3%). A further examination with multivariate logistic regression analyses identified omental transposition as the only independent prognostic factor in intrathoracic infection. A set of criteria were applied and remarkable differences were observed, with the non-transposition group more prone to severe infection. These criteria originated at the 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference (16) and were simplified to facilitate practical use in retrospective analysis. Those on the conference panel suggested caution in interpreting clinical situations because bedside reality was of no less importance (16). Nevertheless, we still hold that these criteria have some value in that they restrict subjective bias from individual clinicians and compensate for unsound diagnoses.
We also looked into patient cost using built-in software in the electronic medical record system of our institution. The data demonstrated a parallel treatment cost between the two groups during hospitalization, not counting health care subsidies. A lower pharmacy cost was observed in the transposition group with a discrepancy of about 4,000 yuan, which was set off to some extent by a significantly higher surgery cost. Given the decline in the incidence and severity of postoperative intrathoracic infection, the decrease in pharmacy cost is presumed to be attributable to reduced antibiotic use in the transposition group, as the latter is a prominent contributor to perioperative cost and is thus greatly influenced by the presence of infection. In contrast, the explanation of the increased surgery cost is subtle, perhaps due to the disposable instruments for the extra technique, but no further conclusions can be reached without access to detailed information. Moreover, the other costs were not stratified, such as examination cost, treatment cost, nursing cost, anesthesia and material cost. Above all, patients receiving omental transposition do not typically endure extra cost and are less exposed to drug toxicity due to reduced pharmacy consumption.
As for anastomotic leakage, our study did not favor mediastinal transposition of the omentum to reduce the incidence of leaks. However, the overall anastomotic leak rate in our series was 6.7%—substantially lower than the 10% reported historically (3), although mortality was as high as 66.6% (2/3) in the non-transposition group. The statistical insignificance could be due to fewer case numbers for each group, and it still can be inferred from the different outcomes for leakage patients in both groups, given the potential role of transposition in containing anastomotic leaks. From these, we speculate that the transposed omentum, which was left relatively motile in the mediastinum, actually enhanced the thoracic cavity’s overall anti-infectious ability rather than protecting anastomosis alone.
Sound surgical skills are also important and should not be dismissed. These include: (I) optimal tension to guarantee sufficient blood supply after the greater omentum is brought into the thorax; (II) expanding the diaphragmatic hiatus to an extent free of both omentum compression and secondary hernia; and (III) a preceding evaluation of the omental graft’s ability to facilitate the removal of degenerated or ischemic omental parts. These technical tips are identical to those in conventional omental wrapping, yet they are crucial to the successful functioning of the transposed omentum. This was testified by Sepesi et al. (12), whose study identified the surgeon variable as an independent predictive factor in anastomotic leakage in addition to omental wrapping.
The limitations of our study lie in part in the nature of retrospective study design. Confounding variables that were hardly measurable might have concealed real statistical significance in the analytic process. The definition of overt intrathoracic infection was revised from an international counterpart, as its validity might be compromised when applied to clinical data. To our knowledge, there is no universal grading system for infection, so these self-revised criteria still provide some objectivity in our opinion. Finally, the aim of the introduced technique is to extend the protective function of conventional omental wrapping to a broader spectrum that covers overall intrathoracic complications. Thus, to further evaluate this technique, an extra group of patients who undergo omental wrapping should be included.
In conclusion, mediastinal transposition of the omentum effectively decreases the rate and severity of postoperative intrathoracic infection following an Ivor-Lewis esophagectomy. This procedure is economically justifiable because the in-hospital cost does not increase while the pharmacy cost is reduced. Our data favored the clinical use of this modification of conventional omental wrapping technique. Nevertheless, prospective randomized studies of larger cohorts are still needed to further investigate the competence of both techniques in postoperative anastomotic leak prevention.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study received approval from the Ethics Committee of The First Affiliated Hospital, School of Medicine, Zhejiang University (No. 2014346). A waiver of consent was granted for this retrospective data review.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Straatman J, Joosten PJ, Terwee CB, et al. Systematic review of patient-reported outcome measures in the surgical treatment of patients with esophageal cancer. Dis Esophagus 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg 1995;169:634-40. [Crossref] [PubMed]
- Patil PK, Patel SG, Mistry RC, et al. Cancer of the esophagus: esophagogastric anastomotic leak--a retrospective study of predisposing factors. J Surg Oncol 1992;49:163-7. [Crossref] [PubMed]
- Müller JM, Erasmi H, Stelzner M, et al. Surgical therapy of oesophageal carcinoma. Br J Surg 1990;77:845-57. [Crossref] [PubMed]
- Dai JG, Zhang ZY, Min JX, et al. Wrapping of the omental pedicle flap around esophagogastric anastomosis after esophagectomy for esophageal cancer. Surgery 2011;149:404-10. [Crossref] [PubMed]
- Martin LW, Swisher SG, Hofstetter W, et al. Intrathoracic leaks following esophagectomy are no longer associated with increased mortality. Ann Surg 2005;242:392-9; discussion 399-402. [PubMed]
- Tocchi A, Mazzoni G, Lepre L, et al. Prospective evaluation of omentoplasty in preventing leakage of colorectal anastomosis. Dis Colon Rectum 2000;43:951-5. [Crossref] [PubMed]
- Zhang K, Yang YH. Use of pedicled omentum in oesophagogastric anastomosis: analysis of 100 cases. Ann R Coll Surg Engl 1987;69:209-11. [PubMed]
- Liu QX, Deng XF, Hou B, et al. Preventing and localizing esophagogastric anastomosis leakage by sleeve-wrapping of the pedicled omentum. World J Gastroenterol 2014;20:16282-6. [Crossref] [PubMed]
- Bhat MA, Dar MA, Lone GN, et al. Use of pedicled omentum in esophagogastric anastomosis for prevention of anastomotic leak. Ann Thorac Surg 2006;82:1857-62. [Crossref] [PubMed]
- Sepesi B, Swisher SG, Walsh GL, et al. Omental reinforcement of the thoracic esophagogastric anastomosis: an analysis of leak and reintervention rates in patients undergoing planned and salvage esophagectomy. J Thorac Cardiovasc Surg 2012;144:1146-50. [Crossref] [PubMed]
- Zheng QF, Wang JJ, Ying MG, et al. Omentoplasty in preventing anastomotic leakage of oesophagogastrostomy following radical oesophagectomy with three-field lymphadenectomy. Eur J Cardiothorac Surg 2013;43:274-8. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Hu Y, Hu C, Zhang H, et al. How does the number of resected lymph nodes influence TNM staging and prognosis for esophageal carcinoma? Ann Surg Oncol 2010;17:784-90. [Crossref] [PubMed]
- Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250-6. [Crossref] [PubMed]
- Morison R. Remarks on some functions of the omentum. Br Med J 1906;1:76-8. [Crossref] [PubMed]
- Law S, Wong KH, Kwok KF, et al. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg 2004;240:791-800. [Crossref] [PubMed]


