Nm23-H1 was involved in regulation of KAI1 expression in high-metastatic lung cancer cells L9981
Introduction
Lung cancer is one of the most prevalent malignancies worldwide, with more than 1 million deaths every year. Invasion and metastasis of tumor cancer is a complex, multi-step progress that numerous cellular genes were involved. Many lung cancer patients develop metastatic diseases, which are the main cause of death (1,2). However, there is less effective method for early detection, and the highly metastatic ability might lead to the mortality of lung cancer.
The metastatic suppressor Nm23-H1 is highly conserved in eukaryotic species, which possess various biological functions, such as transcriptional regulation, proliferation, differentiation, and tumor metastasis suppression. Down-regulation of Nm23-H1 expression level was observed at late stages with high metastatic tumors. It was reported that Nm23-H1 interacts with a number of cellular proteins involved in signaling pathways and oncoproteins of tumor viruses (3,4). Therefore, the elucidation of these interrelationships could useful to study the metastasis suppressor gene ability of Nm23-H1, as well as the function of the partner protein themselves.
The function of KAI1 (CD82) in cancer progression was analyzed using genetic screening in a prostate cancer cell line aim to identify metastasis suppress genes (5). Down-regulation of KAI1 expression was associated with advanced stages of many malignancies including prostate, colon, lung, pancreatic, breast, ovarian and other cancers (6,7). KAI1 belongs to transmembrane 4 superfamily (TM4SF), encoding a 267 amino acid glycosylated membrane protein with four highly conserved transmembrane domain. Previous study revealed the existence of CpG island in the 5’-promoter of KAI1, which extend to the first exon and first intron (8), indicating the possible methylation of KAI1 promoter. It was noted that KAI1 play an important role in cell proliferation, adhesion and movement. KAI1 suppress metastasis through its inhibition of cell movement and adhesion (9,10). Recently, it was reported that no co-localization was observed between a KAI1 splice variant and E-cadherin. Moreover, this KAI1 variant promotes tumor progression and cancer metastasis (11). Besides, KAI1 could regulate MMP-2 and MMP-9 level in patients with endometrial cancer. The tumor development might be influenced by crosstalk between these proteins (12). HBV has been found to inhibit KAI1 by hypermethylation of KAI1 promoter, which maybe a key step in hepatocellular carcinoma progression (13). In addition, there exists a correlation between KAI1 and VEGF expression in the serum or tissue sample in HCC patients (14). The inhibition of KAI1 was related to EGFR over-expression, as well as EGFR mutations in non-small cell lung cancers (15). KAI1 suppress metastasis by inhibition of Wnt signaling pathway through up-regulation of miR-203 and directly down-regulation of FZD2 in lung cancer (16). Thus, it was accepted that KAI1 suppresses tumor metastasis via multiple signaling pathway and considered as a valuable marker for assessing metastasis potential (17). Nonetheless, the regulatory mechanism of KAI1 and its interrelationship with other factors in lung cancer is not fully elucidated.
In this study, we discovered different expression level of KAI1 among diverse lung cancer cells. The results showed a reduction of KAI1 in high metastasis potential L9981 cells. Further we demonstrated inhibition of migration and invasion ability of L9981 by KAI1 transfection. The migration of NL9980, a cell line with low metastatic potential, was enhanced by inhibition of KAI1. Next, p53 was found to activate KAI1 promoter and enhance KAI1 expression. Up-regulation of KAI1 was also observed by over-expression of nm23-H1 in L9981 cells. Moreover, over-expression of p53 and nm23-H1 lead to inhibition of invasion. The motif between −922 and −846 was identified as responsive sites for nm23-H1 in KAI1 promoter. Nevertheless, no obvious methylation of KAI1 promoter was detected by bisulfite sequencing and methylation specific PCR in L9981 and NL9980 cells. The findings of the present study implied a synergistic mechanism of tumor suppressors in lung cancer metastasis.
Methods
Cell culture and transfection
NL9980 and L9981 were isolated and established from a human lung large cell carcinoma cell line (WCQH-9801). The L9981 cells have low expression of nm23-H1 and higher invasion and metastasis potential (18). L9981-nm23-H1 cell line was established by infected virus with nm23-H1 gene (19). Other lung cancer cell lines include A549, GLC-82, A2, SPCA-1, NCI-H460, SH-77, NCI-H446, LTEP-α-2, YTMLC-9 and normal lung cell line MRC-5. Cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (GIBCO, USA) at 37 °C with 5% CO2 incubator. Cell transfection was carried out using Lipo2000 (Invitrogen, CA, USA) according to instruction manual.
Plasmids and siRNA
KAI1 expression plasmid, pCMV-KAI1, were constructed by PCR of full-length KAI1 gene and cloned into pCMV-Tag2B vector. For the construction of pGL3-922, KAI1 promoter sequences from −922 to +353 were amplified from human genomic DNA and cloned into reporter plasmid pGL3-basic. Plasmids pGL3-730 and pGL3-600 were constructed by amplifying promoter sequences from pGL3-922, then cloned the products into pGL3-basic. The primers were listed in Table 1. Plasmid pGL3-922-p53m was constructed as follows: PCR was conducted for the first round with forward primer 5'-TACTTCCCCAGGGGCCAGTCCAGGAGACTTCAGC--CTGT-3' and reverse primer 5'-CCCAAGCTTTGGAATTCACCTGGTTCAC-3' using pGL3-922 as template. Then the PCR product was used as template for the second round of PCR with the forward primer 5'-CTAGCTAGCGTTCTGGGCTACTTCCCCAGGGGCC-3' and reverse primer 5'-CCCAAGCTTTGGAATTCACCTGGTTCAC-3'. The PCR product from the second round was cloned into pGL3-basic. Plasmid pGL3-846 was constructed as follows: DNA fragment of promoter sequence from −846 to +353 was generated by digestion of pGL3-922 with BamH I and Hind III, then the fragment was ligated with Bgl II and Hind III double-digested pGL3-basic vector. All the constructions were verified by sequencing. pEGFP-p53 was purchased from Clontech. KAI1 siRNA was purchased from Ribobio (Guangzhou, China).

Full table
Cell Migration and invasion assays
Cell migration assay was carried out using wound-healing assay. Briefly, cells were cultured in twelve-well plates. Twenty-four hours post transfection, a plastic micropipette tip was used to generate a wound area across the center of the well, and the floating debris were washed with PBS and cultured in serum-free medium. Width of the wound was measured at indicated time points. Three to four wound areas were investigated and photographed under a phase-contrast inverted microscope at 40× magnifications.
The cell invasion ability was determined using BD matrigel invasion chambers according to manufacture’s protocol. Briefly, sixteen hours post transfection, cells were seeded onto upper chambers. After incubation for 48 h at 37 °C, stain of invasion cells was carried out using 0.1% crystal violet after fixation, and five random fields from each insert were counted at 200× magnifications. The invasion assay was conducted in duplicate in two separate experiments.
Quantitative real-time polymerase chain reaction (PCR)
For real-time PCR, 1.0 μg of total RNA was used with hexamer random primers for the first strand cDNA synthesis by MLV Reverse Transcriptase (Takara, Japan). The PCR reaction was carried out using the ABI Prism 7900HT fast RT-PCR system (Applied Biosystems, Life technologies, USA). In each experiment, all the samples were tested in triplicates. The relative amount of mRNA was calculated by the 2-ΔΔCt method after normalization to endogenous GAPDH mRNA levels. Primer sequences to detect KAI1 and GAPDH expression were designed refer to published paper (20).
Antibodies and Western blot
All the antibodies were purchased from Santa Cruz. The cells were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% Nonidet P-40; 0.5% sodium deoxycholate; 0.1% SDS; 1 mM EDTA; 1 mM PMSF; 1 mg/mL aprotinin). Protein concentrations were measured using BCA method. The cell lysates were electrophoresed on 10–15% SDS-PAGE gel, transferred to nitrocellulose membrane. The membranes were blocked with 5% skimmed milk in PBS-Tween (0.1%) for one hour. Then the membranes were probed with antibodies against KAI1, p53 and nm23-H1 at dilution of 1:1,000, and β-actin at dilution of 1:2,000, followed by incubation at 4 °C overnight. After incubation with HRP-conjugated secondary antibody for 1 h, the blots were visualized with Chemiluminescent HRP Substrate (Millipore).
Luciferase assay
L9981 cells were plated in 24-well plate at 1×105 cells/well. The cells were co-transfected with 200 ng of pGL3-922, pGL3-922-p53m or pGL3-Basic, and 100 ng of pEGFP-p53 or pEGFP vector. Co-transfection of L9981 or L9981-nm23-H1 cells was conducted using 200 ng of reporter plasmids with different length of KAI1 promoter. Forty-eight hours after transfection, cells were lysed and assayed for luciferase activity using Dual-luciferase glow assay (Promega, Madison, WI) according to manufacturer’s protocol. All transfection experiments were carried out in triplicate.
Bisulfite sequencing
Genomic DNA was extracted from NL9980 and L9981 cell lines using the Genome DNA extraction kit (Takara) according to manufacturer’s instructions. Bisulfite conversion of 1μg DNA was conducted with Methylamp DNA Modification Kit (Epigentek). The DNA samples were used for the PCR amplification by hot-start Taq DNA polymerase (Takara). Two domains of KAI1 gene were subjected to sequencing. The primers for the first domain (−474 to −190) were 5'-GTAGGGTAGGGTAGGATTAGGAA-3' as sense primer and 5'-ACCAACCTCACCCCCAAACCCAAC-3' as antisense primer. The primers for the second domain (−156 to +126) and PCR condition have been described previously (21). The PCR products were cloned into pMD18T vector (Takara) and subjected to sequencing. Five clones of each domain were selected for sequence analysis.
Methylation-specific polymerase chain reaction
The primers and conditions for the MSP analysis of KAI1 promoter have been described (22). The un-methylated primers were 5'-ATAGAGGAGAGATTTTGTAGT-3' (forward) and 5'-CCCAAAACTCAATCACTCCTA-3' (reverse), the methylated primers were 5'-ATAGAGGAGAGATTTCGTAGC-3' (forward) and 5'-CCGAAACTCAATCACTCCTC-3' (reverse). Bisulfite modified genomic DNA was amplified by MSP at least twice using hot-start Taq DNA polymerase (Takara) and the PCR products were subjected to agarose gel analysis. Controls of non-methylated template and methylated template were purchased from Qiagen.
Statistical analysis
The data were presented as mean ± standard deviation (SD). The statistical comparisons of control and treatment groups were analysis by Student’s t-test. Data processing was carried out using SPSS15.0 software. The differences with P<0.05 were considered statistically significant.
Results
KAI1 is down-regulated in high metastatic lung cancer cells L9981
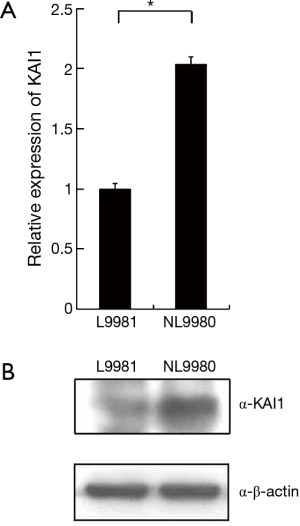
To study the regulatory mechanism of KAI1 in human lung cancer tumorigenesis, KAI1 expression level was detected by real time PCR in different lung cancer cell lines. The data showed in Table 2 indicated that expression level of KAI1 mRNA in different types of lung cancer cell lines is remarkably lower than those in normal human fetal lung fibroblast cell line MRC-5. Moreover, the expression of KAI1 mRNA in the human large cell lung cancer cell lines with opposite metastatic potential is also different. As sub-cell lines with high or low metastatic and invasion ability, we further analyzed the KAI1 expression level in L9981 and NL9980 by real time PCR and western blot assay. Results showed that both mRNA and protein level were lower in L9981 than NL9980 (Figure 1). L9981 is a high metastasis cell line compared with NL9980. Thus, the results here suggested that down-regulation of KAI1 expression maybe related with high metastasis phenotype in human lung cancer cells.
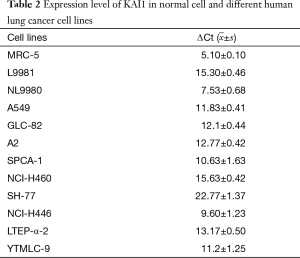
Full table
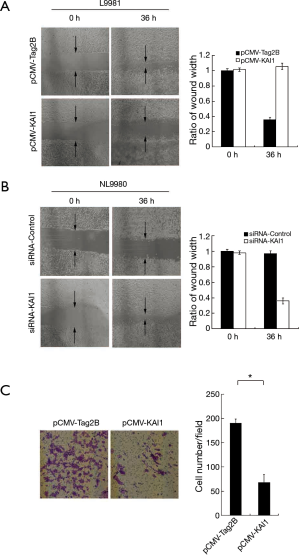
KAI1 suppresses migration and invasion of L9981
To evaluate whether KAI1 suppress metastasis of lung cancer cells L9981, we examined the effect of KAI1 on migration of lung cancer cells by wound healing assay. The L9981 cells were transfected with pCMV-KAI1 or the pCMV-Tag2B vector, and cell migration was detected after 36 h. As shown in Figure 2A, the cell motility was decreased after KAI1 was over-expressed. Moreover, the migration of NL9980 cells was enhanced by inhibition of KAI1 (Figure 2B). To determine another important step in cancer cell metastasis, we analyzed whether KAI1 has influence on cell invasion of L9981, which was assessed by invasion assay. Cells were transfected with pCMV-KAI1 and the vector plasmid, the invasive cells were stained and counted under microscope. In compare with the control group, a significant decrease of invasive cells in KAI1 over-expressed group was observed (Figure 2C). These results were in accordance with the wound healing assay. Collectively, our data implied that over-expression of KAI1 could inhibit progression of high metastasis L9981 cells.
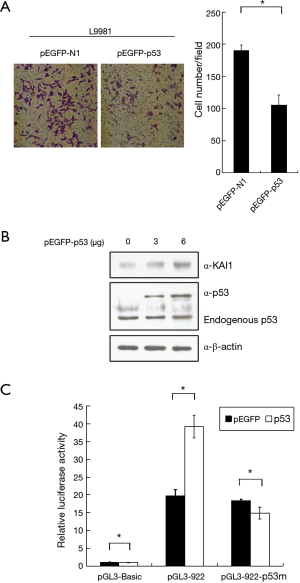
p53 increase KAI1 expression level
Recently, it was noted that KAI1 expression can be regulated by p53 (23). We sought to examine this issue in high-metastatic lung cancer cell lines. The results in Figure 3A suggested that invasion ability of L9981 cells was inhibited by over-expression of p53. Then increasing amounts of pEGFP-p53 expression plasmid were transfected into L9981 cells, and western blot assay was carried out to assess the KAI1 expression level. Results showed in Figure 3B demonstrated that over-expression of p53 can up-regulate KAI1 protein level in L9981. To understand whether KAI1 promoter activity was involved in this up-regulation effect, reporter plasmid containing KAI1 promoter region from −922 to +353 was constructed, which the published p53 binding site was included. Results of luciferase activity assay revealed that KAI1 promoter can be activated by p53. However, mutation of the p53 binding site in KAI1 promoter abolished this activation effect (Figure 3C), indicating the induction of KAI1 expression by p53 in L9981 cells.
Nm23-H1 increase KAI1 expression in L9981 cells
In order to confirm a cross-talk between KAI1 and nm23-H1, L9981-nm23-H1 was established by stable transfection of nm23-H1 into L9981 cells. We discovered that the invasion ability of L9981-nm23-H1 was inhibited compare with L9981 cells (Figure 4A). The mRNA and protein level of KAI1 was measured by real time PCR and western blot in L9981 and L9981-nm23-H1 cells. We found that KAI1 expression, at both mRNA and protein level, is higher in L9981-nm23-H1 than those of L9981 cells (Figure 4B and C). Hence, these results indicated that expression of nm23-H1 lead to up-regulation of KAI1 in L9981-nm23H1 cells.
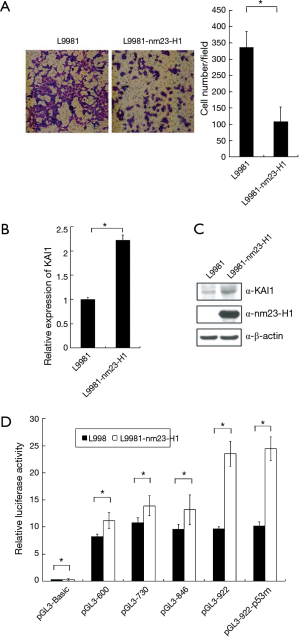
Next, to study whether nm23-H1 alter KAI1 promoter activity, reporter plasmid pGL3-922, which carrying KAI1 promoter, was transfected into L9981 and L9981-nm23-H1 cells. Forty-eight hours post transfection, cells were lysed and luciferase activity was assayed. As shown in Figure 4D, expression of nm23-H1 result in an increase of luciferase activity in L9981-nm23-H1 compared with L9981 cells, which indicated that KAI1 reporter activity can be induced by expression of nm23-H1. To find the promoter domain responsible for nm23-H1 activation, we constructed a series of reporter plasmids with different length of KAI1 promoter. L9981 and L9981-nm23-H1 cells were transfected with these reporter plasmids. The results of luciferase assay demonstrated that only pGL3-922 is able to induced by nm23-H1 expression (Figure 4D), while activation of other truncated promoters (pGL3-846,730 and 600) was weakly detectable. Therefore, it was probable that the responsive element for nm23-H1 in KAI1 promoter was between −922 to −846. Interestingly, p53 binding site is dispensable for nm23-H1-mediated stimulation of KAI1 promoter (Figure 4D), which showed that KAI1 can be activated by nm23-H1 without intact p53 binding site.
Methylation is not significant for KAI1 expression in L9981 cells
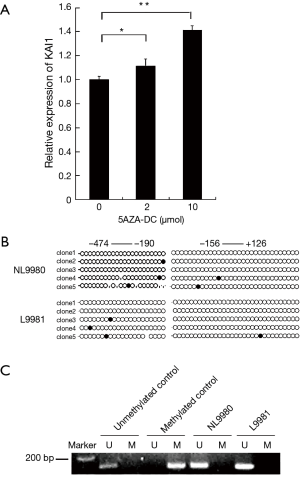
It was unclear whether KAI1 promoter activity was regulated by methylation in L9981 cells, thus we applied 5-AZA-DC, a methyltransferase inhibitor, to analyze KAI1 expression. We observed restoration of KAI1 level by 5-AZA-DC treatment in L9981 cells (Figure 5A). This result indicated that promoter methylation might be involved in the down-regulation of KAI1 in lung cancer cells. Next, the methylation status of KAI1 promoter was studied using bisulfite sequencing and methylation specific PCR (MSP). Two domains, −474 to −190 and −156 to 126 of KAI1 promoter, were subjected to bisulfite sequencing. However, results revealed that CpG island in KAI1 promoter does not exhibit high methylation level in L9981 or NL9980 cells (Figure 5B). Correlate with results of bisulfite sequencing, no methylation was observed in MSP analysis (Figure 5C). Taken together, it thus implicates that KAI1 expression maybe mediated through other pathway, rather than methylation of promoter in these two cell lines.
Discussion
Although many breakthroughs were achieved for early detection and personalized therapy of lung cancer, it is still the leading cause of death in the world. Notably, primary cancer may not be the reason for the death, whereas a majority of patients die of tumor metastasis. As a complicated and multi-level biological process, further research is required to study the molecular mechanism of metastasis in lung cancer. It was known that metastasis suppressors exert their functions by inhibiting motility and invasiveness of tumor cells. Aside from manipulating the cellular affinity of tumor alone, co-operation of different suppressors may occur to inhibit cancer metastasis.
It was recently reported that KAI1 and KISS1 (a metastasis suppressor gene) expression was significantly decreased in breast cancer in comparison with normal tissue, especially in patients with aggressive tumors (24). Moreover, Wu et al. observed a positive relation between KAI1 and E-cadherin expression in NSCLC, and down-regulation of these two proteins was closely related to differentiation, pTNM stages and metastasis (25). The study on the expression of HIF-1α and VEGF demonstrated that they were negatively regulated by KAI1 in the human prostate cancer cell line PC3, which contribute to inhibition of cancer metastasis (26). Besides, KAI1 was activated by recruiting histone acetyltransferase Tip60 in the presence of PMA (27). Therefore, KAI1 exerts profound metastasis-suppressor activity in the tumor malignancy process.
Previous reports have proved that KAI1 expression is down-regulated in many cancers. Hence, we compared KAI1 expression in various lung cancer cells, and a pair of high and low metastatic potential cell lines (L9981 and NL9980). The results showed decreased mRNA and protein level of KAI1 in L9981 cells compared to NL9980 cells. These data correlate well with the founding that expression of KAI1 was down-regulated in several cancers with advanced stage, including ovarian, colon and lung (7). There was evidence revealed that KAI1 and nm23-H1 were down-regulated simultaneously in tumor progression of non-small cell lung cancer (28). Besides, KAI1 expression was decreased in basal cell carcinomas, while nm23-H1 was up-regulated (29). It was reported that expression of KAI1 and nm23-H1 were correlated with prognoses of pancreatic cancer and papilla of vater cancer (30). Thus we detected KAI1 level in L9981 and L9981-nm23-H1, a stable nm23-H1 expression cell line. The results implied that KAI1 expression can be up-regulated by nm23-H1. Because low level of nm23-H1 is frequently observed in the progression of several cancers (31,32), our results indicated that loss expression of nm23-H1 might contribute to the down-regulation of KAI1.
To identify the responsive motif for nm23-H1 in KAI1 promoter, a series of plasmids with different length of KAI1 promoter were constructed and reporter assay were carried out. Interestingly, we found that nm23-H1 may bind to the KAI1 promoter between −922 to −846, a domain that probably carrying AP1, AP2 and p53 binding sites (33). It has been reported that nm23-H1 was positively regulated by p53 via removing of Mdm2, a negative regulator of p53, from p53-Mdm2 complex (34), and KAI1 expression can be up-regulated by p53 (35). Results of invasion assay showed an inhibition of L9981 invasive ability by over-expression of p53. We further demonstrated that p53 can up-regulated KAI1 expression through promoter activation in L9981 cells. These data indicate the possibility that p53 promote the expression of KAI1, then lead to the inhibition of invasion in L9981 cells. By contrast, we found p53 binding site is not indispensable for KAI1 promoter induction by nm23-H1, which indicated that nm23-H1 activate KAI1 promoter in a p53-independent manner in L9981 cells.
The methylated modification of promoter contributes to loss of expression of several tumor suppressors. High methylation status of KAI1 was observed in myeloma cell lines and the methyltransferase inhibitor 5’-aza-CdR is able to promote KAI1 expression in U266 cells (22). There are also reports suggested KAI1 gene was unmethylated in oral cancer and melanoma cells (21,36). In the present study, only low level of methylation could be detected in L9981 and NL9980 cells by bisulfite sequencing, while the MSP assay demonstrated non-methylation of KAI1 promoter in these two cell lines. There were reports from several groups suggested that methylation may not the key mechanism for reduction of KAI1 expression, which suggest that other pathway was involved in KAI1 regulation (21,37,38). Taken together, these result implicated that KAI1 was not methylated at least in cancer cell NL9980 or L9981. Though we found KAI1 expression can be up-regulated by 5-AZA-dc, this might be an indirect effect.
In conclusion, the results of present study confirmed the suppressor activity of KAI1 in high metastasis potential L9981 cells, as well as suggested the positive relationship between KAI1 and nm23-H1 in lung cancer. We also showed that KAI1 promoter was activated by nm23-H1 in a p53-independent fashion. There should be more research aim to further analyze the interaction between KAI1 and nm23-H1. Moreover, the mechanism of synergistic inhibition of cancer metastasis by KAI1 and other metastasis suppressors is worth studying. If such a crosstalk was discovered between KAI1 signaling and pathways of other metastasis suppressors, new targets will be identified that helpful to the cancer therapy.
Acknowledgements
Funding: This study was partly supported by the grants from the Key Project from National Natural Science Foundation of China (No. 81000950), National 863 Program (No. 2012AA02A201, No. 2012AA02A502), National 973 Program (No. 2010CB529405), and TMUGH funding (No. ZYYFY2014002).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Travis WD. Pathology of lung cancer. Clin Chest Med 2002;23:65-81. viii. [Crossref] [PubMed]
- Wang X, Ling C, Bai Y, et al. MicroRNA-206 is associated with invasion and metastasis of lung cancer. Anat Rec (Hoboken) 2011;294:88-92. [Crossref] [PubMed]
- Marino N, Marshall JC, Steeg PS. Protein-protein interactions: a mechanism regulating the anti-metastatic properties of Nm23-H1. Naunyn Schmiedebergs Arch Pharmacol 2011;384:351-62. [Crossref] [PubMed]
- Saha A, Robertson ES. Functional modulation of the metastatic suppressor Nm23-H1 by oncogenic viruses. FEBS Lett 2011;585:3174-84. [Crossref] [PubMed]
- Ichikawa T, Ichikawa Y, Isaacs JT. Genetic factors and suppression of metastatic ability of prostatic cancer. Cancer Res 1991;51:3788-92. [PubMed]
- Lee HA, Park I, Byun HJ, et al. Metastasis suppressor KAI1/CD82 attenuates the matrix adhesion of human prostate cancer cells by suppressing fibronectin expression and β1 integrin activation. Cell Physiol Biochem 2011;27:575-86. [Crossref] [PubMed]
- Tonoli H, Barrett JC. CD82 metastasis suppressor gene: a potential target for new therapeutics? Trends Mol Med 2005;11:563-70. [Crossref] [PubMed]
- Dong JT, Isaacs WB, Barrett JC, et al. Genomic organization of the human KAI1 metastasis-suppressor gene. Genomics 1997;41:25-32. [Crossref] [PubMed]
- Jee B, Jin K, Hahn JH, et al. Metastasis-suppressor KAI1/CD82 induces homotypic aggregation of human prostate cancer cells through Src-dependent pathway. Exp Mol Med 2003;35:30-7. [Crossref] [PubMed]
- Bari R, Zhang YH, Zhang F, et al. Transmembrane interactions are needed for KAI1/CD82-mediated suppression of cancer invasion and metastasis. Am J Pathol 2009;174:647-60. [Crossref] [PubMed]
- Upheber S, Karle A, Miller J, et al. Alternative splicing of KAI1 abrogates its tumor-suppressive effects on integrin αvβ3-mediated ovarian cancer biology. Cell Signal 2015;27:652-62. [Crossref] [PubMed]
- Grybos A, Bar J. The relationships between the immunoexpression of KAI1, MMP-2, MMP-9 and steroid receptors expression in endometrial cancer. Folia Histochem Cytobiol 2014;52:187-94. [Crossref] [PubMed]
- Yu G, Bing Y, Li W, et al. Hepatitis B virus inhibits the expression of CD82 through hypermethylation of its promoter in hepatoma cells. Mol Med Rep 2014;10:2580-6. [PubMed]
- Zhang W, Zhao CG, Sun HY, et al. Expression characteristics of KAI1 and vascular endothelial growth factor and their diagnostic value for hepatocellular carcinoma. Gut Liver 2014;8:536-42. [Crossref] [PubMed]
- Yang CH, Chou HC, Fu YN, et al. EGFR over-expression in non-small cell lung cancers harboring EGFR mutations is associated with marked down-regulation of CD82. Biochim Biophys Acta 2015;1852:1540-9.
- Mine M, Yamaguchi K, Sugiura T, et al. miR-203 Inhibits Frizzled-2 Expression via CD82/KAI1 Expression in Human Lung Carcinoma Cells. PLoS One 2015;10:e0131350. [Crossref] [PubMed]
- Zhou L, Yu L, Wu S, et al. Clinicopathological significance of KAI1 expression and epithelial-mesenchymal transition in non-small cell lung cancer. World J Surg Oncol 2015;13:234. [Crossref] [PubMed]
- Zhou Q, Wang Y, Che G, et al. Establishment and their biological characteristics of clonal cell subpopulations (NL9980 and L9981) from a human lung large cell carcinoma cell line (WCQH-9801). Zhongguo Fei Ai Za Zhi 2003;6:464-8. [PubMed]
- Che L, Zhou Q, Wang Y, et al. Establishment of a human large cell lung cancer cell line L9981-nm23-H1. Zhongguo Fei Ai Za Zhi 2004;7:187-90. [PubMed]
- Liu FS, Chen JT, Dong JT, et al. KAI1 metastasis suppressor gene is frequently down-regulated in cervical carcinoma. Am J Pathol 2001;159:1629-34. [Crossref] [PubMed]
- Kim YI, Shin MK, Lee JW, et al. Decreased expression of KAI1/CD82 metastasis suppressor gene is associated with loss of heterozygosity in melanoma cell lines. Oncol Rep 2009;21:159-64. [PubMed]
- Drucker L, Tohami T, Tartakover-Matalon S, et al. Promoter hypermethylation of tetraspanin members contributes to their silencing in myeloma cell lines. Carcinogenesis 2006;27:197-204. [Crossref] [PubMed]
- Mashimo T, Watabe M, Hirota S, et al. The expression of the KAI1 gene, a tumor metastasis suppressor, is directly activated by p53. Proc Natl Acad Sci U S A 1998;95:11307-11. [Crossref] [PubMed]
- Mooez S, Malik FA, Kayani MA, et al. Expressional alterations and transcript isoforms of metastasis suppressor genes (KAI1 and KiSS1) in breast cancer patients. Asian Pac J Cancer Prev 2011;12:2785-91. [PubMed]
- Shiwu WU, Lan Y, Wenqing S, et al. Expression and clinical significance of CD82/KAI1 and E-cadherin in non-small cell lung cancer. Arch Iran Med 2012;15:707-12. [PubMed]
- Park JJ, Jin YB, Lee YJ, et al. KAI1 suppresses HIF-1α and VEGF expression by blocking CDCP1-enhanced Src activation in prostate cancer. BMC Cancer 2012;12:81. [Crossref] [PubMed]
- Rowe A, Weiske J, Kramer TS, et al. Phorbol ester enhances KAI1 transcription by recruiting Tip60/Pontin complexes. Neoplasia 2008;10:1421-32, following 1432.
- Goncharuk VN, del-Rosario A, Kren L, et al. Co-downregulation of PTEN, KAI-1, and nm23-H1 tumor/metastasis suppressor proteins in non-small cell lung cancer. Ann Diagn Pathol 2004;8:6-16. [Crossref] [PubMed]
- Bozdogan O, Yulug IG, Vargel I, et al. Differential expression patterns of metastasis suppressor proteins in basal cell carcinoma. Int J Dermatol 2015;54:905-15. [Crossref] [PubMed]
- Friess H, Guo XZ, Tempia-Caliera AA, et al. Differential expression of metastasis-associated genes in papilla of vater and pancreatic cancer correlates with disease stage. J Clin Oncol 2001;19:2422-32. [PubMed]
- Jiang Z, Wang X. Expression and significance of PTEN and nm23-H1 in the metastasis of non-small cell lung cancer. Zhongguo Fei Ai Za Zhi 2008;11:793-7. [PubMed]
- Tee YT, Chen GD, Lin LY, et al. Nm23-H1: a metastasis-associated gene. Taiwan J Obstet Gynecol 2006;45:107-13. [Crossref] [PubMed]
- Marreiros A, Czolij R, Yardley G, et al. Identification of regulatory regions within the KAI1 promoter: a role for binding of AP1, AP2 and p53. Gene 2003;302:155-64. [Crossref] [PubMed]
- Jung H, Seong HA, Ha H. NM23-H1 tumor suppressor and its interacting partner STRAP activate p53 function. J Biol Chem 2007;282:35293-307. [Crossref] [PubMed]
- Marreiros A, Dudgeon K, Dao V, et al. KAI1 promoter activity is dependent on p53, junB and AP2: evidence for a possible mechanism underlying loss of KAI1 expression in cancer cells. Oncogene 2005;24:637-49. [Crossref] [PubMed]
- Uzawa K, Ono K, Suzuki H, et al. High prevalence of decreased expression of KAI1 metastasis suppressor in human oral carcinogenesis. Clin Cancer Res 2002;8:828-35. [PubMed]
- Jackson P, Millar D, Kingsley E, et al. Methylation of a CpG island within the promoter region of the KAI1 metastasis suppressor gene is not responsible for down-regulation of KAI1 expression in invasive cancers or cancer cell lines. Cancer Lett 2000;157:169-76. [Crossref] [PubMed]
- Sekita N, Suzuki H, Ichikawa T, et al. Epigenetic regulation of the KAI1 metastasis suppressor gene in human prostate cancer cell lines. Jpn J Cancer Res 2001;92:947-51. [Crossref] [PubMed]


