
Pirfenidone and nintedanib attenuate pulmonary fibrosis in mice by inhibiting the expression of JAK2
Highlight box
Key findings
• In this study, two pathways, JAK2/STAT3 and transforming growth factor-β, were found to interact with each other through anti-fibrotic drugs and to be jointly involved in the development of pulmonary fibrosis.
What is known and what is new?
• Pirfenidone and nintedanib have antifibrotic effects.
• We identified the mechanism of action of these two antifibrotic drugs in bleomycin-induced lung fibres in mice.
What is the implication, and what should change now?
• We can look at different pathway mechanisms to find the most appropriate way to reduce the extent of pulmonary fibrosis.
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common type of interstitial lung disease (ILD). IPF is most common among men and among people over the age of 50 years. The disease course of IPF is variable and unpredictable, with a median survival of only 2–4 years after diagnosis (1). The intrapulmonary manifestation of IPF is that activated cells in the alveoli release numerous cytokines and growth factors, which promote the recruitment, proliferation and differentiation of lung fibroblasts into myofibroblasts, resulting in excessive collagen deposition, progressive scarring of the lung parenchyma, and irreversible loss of lung function (2). IPF affects three million people worldwide. The currently available antifibrotic drugs include pirfenidone and nintedanib. Although these two drugs can slow disease progression, they cannot improve or stabilize lung function and may cause intolerance issues (3).
Pirfenidone is an oral pyridine that exhibits anti-inflammatory, antioxidant and antifibrotic effects in both cell and animal experiments, including modulation of transforming growth factor-β (TGF-β) expression and inhibition of fibroblast and collagen synthesis (4-7). Nintedanib is an inhibitor of multiple tyrosine kinase receptors involved in the pathogenesis of pulmonary fibrosis, for example, platelet-derived growth factor (PDGF) receptors α and β; vascular endothelial growth factor (VEGF) receptors 1, 2, and 3; and fibroblast growth factor (FGF) receptors 1, 2, and 3. Nintedanib has been shown to prevent the development of pulmonary fibrosis in the bleomycin (BLM) mouse model (8-11). Although pirfenidone and nintedanib are Food and Drug Administration (FDA)-approved for the treatment of IPF, their mechanisms of action are not fully understood (12).
TGF-β is a multifunctional cytokine with three subtypes: TGF-β1, TGF-β2, and TGF-β3. TGF-β1 plays a major role in pulmonary fibrosis. TGF-β1 is a potent profibrotic mediator that promotes epithelial-mesenchymal transition (EMT), epithelial cell apoptosis, epithelial cell migration, and the production of other profibrotic mediators (13). In fibrosis, TGF-β1 promotes the activation of Smad2/3 by binding and activating its receptor complexes (TβRI and TβRII). Activated Smad2/3 forms a complex with common partner Smads, Smad4; this complex enters the nucleus and binds certain Smad-binding sequences in the promoter regions of target genes, including fibronectin, collagen and plasminogen activator inhibitor-1 (PAI-1), promoting their transcription. Furthermore, TGF-β1 signaling induces fibrosis not only through Smad-dependent canonical pathways but also through Smad-independent noncanonical pathways, such as the JAK/STAT, Ras-MAPK, TAK1-p38/JNK, PI3K-Akt, and Par6-Smurf1 pathways (14,15).
The JAK/STAT signaling pathway is crucial for cell homeostasis and plays important roles in tumors, inflammation and autoimmune diseases. It is also a pathway involved in ILD (16,17). The JAK2/STAT3 pathway is activated in a variety of fibrotic diseases, such as bone marrow, skin, liver, myocardial and kidney fibrosis (18). In addition to the Smad pathway, TGF-β1 can also induce the phosphorylation of JAK2 through noncanonical pathways. JAK2 interacts with STAT3 and phosphorylates the latter to induce fibrosis (16,18). In addition, JAK2 can be activated by other profibrotic mediators, including PDGF, VEGF, interleukin (IL)-6, IL-13, angiotensin II (ANGII), serotonin (5-HT), and endothelin-1 (ET-1) (18,19). Previous study has reported the presence of STAT3 phosphorylation in fibrotic lung tissue from IPF patients and indicated that STAT3 phosphorylation is involved in the transition from fibroblasts to myofibroblasts and causing damage to lung epithelial cells (19). We hypothesized that pirfenidone and nintedanib can inhibit the expression of JAK2 through the TGF-β1 signaling pathway, thereby attenuating pulmonary fibrosis. The results from cell experiments indicated that there may be mutual influence between the TGF-β1 signaling pathway and the JAK2 signaling pathway. We present this article in accordance with the ARRIVE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1057/rc).
Methods
Animals and cells
Animals
Forty female C57BL/6 mice (body weight: 18–20 g; age: 8 weeks) were purchased from Chengdu Dossy Experimental Animals Co., Ltd. (Chengdu, China) and raised at a temperature of 20–26 °C. The animals had free access to food and water. Experiments were started after one week of adaptive feeding with standard chow. BLM (Takasaki Plant, Nippon Kayaku Co., Ltd., Japan), pirfenidone (AdooQ Bioscience, Nanjing, China), and nintedanib (AdooQ Bioscience) were used for subsequent in vivo experiments.
Cells
Mouse lung epithelial cells (MLE12; Procell Life Science & Technology Co., Ltd., Wuhan, China) were cultured in F12 medium (Shanghai Fuheng Biotechnology Co., Ltd., Shanghai, China) containing 10% fetal bovine serum (FBS) (Biochannel Company, Nanjing, China) under 37 °C and 5% CO2, and cells at passages 3–5 were used for the experiments. TGF-β1 (PeproTech, USA), LY2109761 (a TGF-β1 receptor inhibitor; AdooQ Bioscience), and si-JAK2 (Guangzhou RiboBio Co., Ltd., Guangzhou, China) were used for subsequent in vitro experiments. Experiments were performed under a project license (No. Dossy20210119001) granted by the Ethics Committee of Chengdu Dashuo Biotechnology Co. (Chengdu, China), in compliance with institutional guidelines for the care and use of animals.
Animal modeling and intervention
After 40 8-week-old SPF female C57BL/6 mice were adaptively reared for one week, 10 mice were randomly selected as the control group, and the remaining 30 mice were intratracheally injected with BLM (5 mg/kg) on the first day to generate a pulmonary fibrosis model. On the third day, the 30 mice were randomly divided into three groups, with 10 mice in each group: BLM group (0.1 mL CMC-Na), pirfenidone group (20 mg/kg), and nintedanib group (60 mg/kg) (20,21). After the mice in each group were intragastrically administered the corresponding drug every other day until the 28th day, they were anesthetized and sacrificed for the collection of lung tissue and peripheral plasma.
Enzyme-linked immunosorbent assay (ELISA)
The samples taken for this experiment were serum from mice. ELISAs were used to detect TGF-β1, SP-A, SP-D and KL-6, strictly following the instructions of the ELISAs kit. ELISAs kits were purchased from Jingmei Biotechnology (Jiangsu, China) to complete the experiments. All experimental steps were carried out according to the instructions.
Histological and immunohistochemical analyses
Lung tissue was fixed with 4% formalin for 12–16 h, dehydrated, and embedded in paraffin. The specimen blocks were cut into 5-µm-thick sections. The sections were stained with hematoxylin-eosin (H&E) to observe inflammatory infiltration and the structural integrity of the alveoli. Masson staining was used to assess the extent of collagen deposition.
For immunohistochemical staining of α-smooth muscle actin (α-SMA) and collagen I, tissue slides were deparaffinised with xylene, polarised with decreasing concentrations of alcohol and rinsed with deionised water. Endogenous peroxidase activity was blocked by incubating the sections in 3% hydrogen peroxide solution for 30 min at room temperature after antigen repair. The slides were closed with 10% normal goat serum for 1 hour. The primary antibody was incubated overnight at 4 °C and then the secondary antibody was incubated for 60 min at room temperature. The slides were visualised by diaminobenzidine (DAB) staining and restained with hematoxylin. Integral optical density (IOD) and positive area (area) were quantified by Image J. The mean optical density (MOD = IOD/area) was calculated to evaluate the expression of α-SMA and collagen I.
Western blotting
Total tissue protein was extracted from cell lysates (Beyotime, Shanghai, China), and the protein concentration was determined using a bicinchoninic acid (BCA) protein quantification kit (Beyotime). Protein samples were electrophoresed on sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred to PVDF membranes. The membranes were blocked with 5% nonfat milk powder at room temperature for 2 h, incubated with appropriately diluted primary antibody at 4 °C overnight, rinsed 3 times, and incubated with horseradish peroxidase (HRP)-labeled secondary antibody at room temperature for 2 h. The bands were visualized with a fluorescence chemiluminescence imaging system (Clinx Science Instruments, Shanghai, China). Anti-TGF-β1, anti-α-SMA, anti-Fn, anti-GAPDH, anti-JAK2, anti-p-JAK2, anti-STAT3, and anti-p-STAT3 monoclonal antibodies were purchased from Affinity (Jiangsu, China). Anti-TGF-β-R2 antibodies were purchased from Proteintech. Antibody dilutions were purchased from Beyotime. HRP-labeled goat anti-rabbit and goat anti-mouse IgG antibodies were purchased from EarthOx (USA).
Immunofluorescence
Mouse lung tissue sections and treated cells were collected and blocked with serum. Anti-p-JAK2 antibody (1:50) was added to the sections and cells, which were then incubated at 4 °C overnight in the dark. Then, fluorescent secondary antibodies [1:100, fluorescein isothiocyanate (FITC)-labeled, green] were added, and the sections and cells were placed in a wet box in an incubator at 37 °C for 1 h. Afterwards, 4',6-diamidino-2-phenylindole (DAPI) was added dropwise, and the sections and cells were incubated in the dark for 5 min. The specimens were mounted with anti-fluorescence quenching agent and observed and photographed under a fluorescence microscope.
Statistical analysis
One-way analysis of variance (ANOVA) was performed using GraphPad Prism 9.0 software. The data were presented as the mean ± standard deviation or mean ± standard error. ANOVA was used to analyze differences among multiple groups, and the independent t test was used to analyze differences between two groups. P<0.05 indicated that a difference was statistically significant.
Results
Effects of pirfenidone and nintedanib on plasma biomarkers in mice
We evaluated the therapeutic effect of pirfenidone and nintedanib in mouse pulmonary fibrosis by detecting the levels of TGF-β1, SP-A, SP-D and KL-6 in mouse plasma. Figure 1A shows that the levels of TGF-β1, KL-6, SP-A and SP-D in the plasma of the pulmonary fibrosis in the mouse model were significantly increased in the BLM group. In the pirfenidone and nintedanib groups, the elevations in TGF-β1, KL-6, SP-A and SP-D levels in the plasma were reversed by pirfenidone and nintedanib.
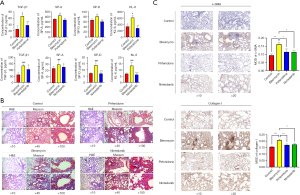
Pirfenidone and nintedanib attenuated BLM-induced pulmonary fibrosis in mice by inhibiting the expression of JAK2
Figure 1B shows H&E and Masson staining of lung tissue from mice sacrificed on day 28. Regarding the H&E results, the mice in the control group had relatively intact lung tissue structures, thin alveolar septa, and a few inflammatory cells in the alveolar septa; the mice in the BLM group had severely damaged lung tissue structures, markedly thickened alveolar septa, considerable inflammatory cell infiltration, and evident inflammatory responses; and the mice in the pirfenidone and nintedanib groups showed less alveolar structure damage and inflammatory cell infiltration than those in the BLM group. Regarding the Masson staining results, there was no obvious blue staining of lung tissue in the control group. The lung tissue in the BLM group was severely damaged, with a large area of blue staining, indicating the deposition of a large number of collagen fibers. Inflammation and fibrosis in the lung tissue of mice in the pirfenidone and nintedanib groups were attenuated compared with those in the lung tissue of mice in the BLM group.
α-SMA is a recognised biomarker of myofibroblast activation and collagen I is an important factor for ECM deposition in the lung interstitium. Figure 1C shows the expression of α-SMA and collagen I in the alveoli and interstitium of the lung observed by immunohistochemistry. The results indicated that α-SMA and collagen I were highly expressed in the interstitium in the BLM group, and the expression of α-SMA and collagen I was significantly reduced in the drug-treated group compared with the BLM group.
To determine whether nintedanib and pirfenidone can reduce pulmonary fibrosis in mice by inhibiting JAK2 expression, Western blotting was performed (Figure 2A). The results indicated that the expression of JAK2, p-JAK2 and p-STAT3 was significantly increased in the BLM group and that intervention with pirfenidone and nintedanib significantly reversed the BLM-induced elevations in these proteins. The expression of TGF-β-R2, SMA (a myofibroblast activation marker) and extracellular matrix (ECM) fibronectin in the BLM group increased, and intervention with pirfenidone and nintedanib reversed the BLM-induced elevations in these proteins. Hence, pirfenidone and nintedanib may inhibit the expression of JAK2 by downregulating the TGF-β1 signaling pathway, thereby attenuating pulmonary fibrosis.
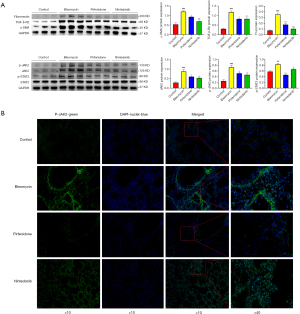
Expression and location of p-JAK2 in mouse lung tissue
As shown in Figure 2B, p-JAK2 was located in the nucleus within the fibrotic areas in BLM-induced mouse lung tissue, and the fluorescence signal of p-JAK2 in the fibrotic areas of BLM-induced mouse lung tissue significantly decreased after pirfenidone and nintedanib treatment. Hence, JAK2 can not only translocate to the nucleus and activate the transcription of target genes through downstream dimerisation of STAT3, but also has the potential to enter the nucleus through phosphorylation and play a role independent of STAT3.
TGF-β1 stimulated JAK2 expression in MLE12 cells
Repeated injury of the alveolar epithelium is the main mechanism for the occurrence of IPF. The injured epithelial cells produce numerous cytokines and promote fibrosis. The development of fibrosis involves complex signaling interactions between epithelial and mesenchymal cells. The active TGF-β1 signaling pathway in tissue remodeling has been shown to play an important role in fibrosis. Therefore, we stimulated MLE12 cells with TGF-β1 to construct an epithelial cell injury model.
TGF-β1 induces the phosphorylation of JAK2, which interacts with STAT3 and phosphorylates the latter, promoting fibrosis. In addition, JAK2 can be activated by other profibrotic factors, including PDGF, VEGF, IL-6, IL-13, ANGII, 5-HT, and ET-1. Figure 3A shows the expression of JAK2, p-JAK2, p-STAT3, TGF-βR2, SMA, and fibronectin after stimulation of MLE12 cells with different concentrations of TGF-β1 (0, 5, 10, 20, 50, and 100 ng/mL). The expression of these proteins was highest with 50 ng/mL TGF-β1, whereas 100 ng/mL TGF-β1 inhibited the expression of these proteins. To further verify whether TGF-β1 increases JAK2 expression, we treated MLE12 cells with different concentrations of the TGF-β1 receptor inhibitor LY2109761 (0.01, 0.05 and 0.5 µM) and observed the concentration-dependent effect of LY2109761 on JAK2. As shown in Figure 3B, with increasing LY2109761 concentrations, the protein expression of JAK2, p-JAK2 and p-STAT3 gradually decreased. Thus, TGF-β1 can stimulate JAK2 expression in MLE12 cells.

Downregulation of JAK2 expression affected the TGF-β1 signaling pathway
To further explore the role of JAK2 in EMT, we observed changes in α-SMA by downregulating the expression of JAK2 (through siRNA or inhibitor treatment). As shown in Figure 3C, after downregulating the expression of JAK2, TGF-βR2 and SMA levels significantly decreased. Hence, JAK2 can affect the TGF-β1 signaling pathway, suggesting that there may be mutual influence between the JAK2 and TGF-β1 pathways. We treated MLE12 cells with pirfenidone (1 mM) and nintedanib (1 µM), as shown in Figure 4A. JAK2, p-JAK2, p-STAT3 and SMA expression in all treated cells significantly decreased. Therefore, in the in vitro experiments, we demonstrated that pirfenidone and nintedanib may attenuate fibrosis by inhibiting JAK2.

JAK2 may function before STAT3 enters the nucleus
In in vivo experiments, we observed p-JAK2 in the nuclei of cells in fibrotic areas in mice with pulmonary fibrosis and speculated that p-JAK2 might also be located in the nucleus in cellular experiments. We treated MLE12 cells with TGF-β1 (50 ng/mL) at different time points (0, 1, 2, 6, 12, 24 h) and found that the expression of p-JAK2 increased after treatment at 1 h, decreased after treatment at all time points between 2 and 12 h, and increased after treatment at 24 h (Figure 4B). The expression of p-STAT3 was increased after treatment at 2 h, then gradually decreased before increasing again at 24 h. Therefore, we speculate that JAK2 may play a role before STAT3 enters the nucleus.
Discussion
In recent years, increasing evidence has shown that IPF is an epithelial injury-driven disease and that abnormally activated alveolar epithelium can lead to the migration, proliferation, and differentiation of fibroblasts into myofibroblasts. These myofibroblasts secrete large amounts of ECM, leading to lung structure remodeling. At present, despite its limitations, the BLM-induced pulmonary fibrosis model is still a popular model for exploring the pathogenesis of IPF and the effectiveness of new antifibrotic drugs (22,23). In this study, we established a BLM-induced lung fibrosis model in mice and investigated the possible mechanisms of action of two antifibrotic drugs, pirfenidone and nintedanib. MLE12 cells, a widely used mouse capillary alveolar epithelial cell line, were also selected for this experiment, and there is a large body of literature using MLE12 cells to study the biological properties of alveolar epithelial cells and the mechanisms of related diseases (24-26).
Among the animal models of pulmonary fibrosis, in addition to BLM-induced pulmonary fibrosis, they include fluorescein isothiocyanate, silica, and radiation (27) as well as the injury model of butylated hydroxytoluene (BHT) proposed (28). The BHT model is a model with distal alveolar involvement, and the pathology is much closer to that of human pulmonary fibrosis. In contrast, the histology of the BLM animal model shows a proximal airway-centred pattern similar to human airway-centred interstitial pneumonia. However, there is no literature available to suggest whether the molecular mechanisms of signalling that they ultimately lead to pulmonary fibrosis are different. The BLM model is the most widely used and best characterised mouse model. The BLM-induced pulmonary fibrosis model can be readily induced in a short period of time with high reproducibility (29). Therefore, the BLM model was used in this experiment. Although the model still suffers from resorption of pulmonary fibrosis in mice after 30 days of evolution, the model system provides an opportunity to study the regression of fibrosis at these later time points (29).
Due to the variable and unpredictable course of IPF and the lack of easily reproducible surrogate endpoints for patient-related outcomes, molecular biomarkers are urgently needed to improve the prognosis of this chronic lung disease. Therefore, it is of great value to identify easily detectable specific serum biomarkers. Recent studies have reported that peripheral blood can be used to indicate the presence, stage, and prognosis of IPF (30,31). When alveolar epithelial cells are damaged, KL-6, SP-A and SP-D are released from these cells and can be detected in blood. Previous study has suggested that TGF-β1 is involved in fibrotic diseases. This molecule is a fibroblast chemokine that can recruit fibroblasts in surrounding tissues to aggregate, proliferate and differentiate into damaged areas, produce a large amount of collagen, and stimulate the synthesis and secretion of various cytokines (32). KL-6 is a mucin-like glycoprotein expressed on the extracellular surfaces of alveolar type II cells and bronchiolar epithelial cells. As a chemokine, KL-6 promotes the migration, proliferation and survival of lung fibroblasts. SP-A and SP-D are also produced by type II alveolar epithelial cells, and together, the two participate in the formation of alveolar surfactants and in the formation and metabolism of alveolar hyaline membranes (33-36). We detected biomarkers (KL-6, SP-A, and SP-D) in mouse plasma by ELISAs and found that the expression of these markers after pirfenidone and nintedanib treatment was significantly lower than that in the BLM group, suggesting that these biomarkers can potentially be used as indicators of therapeutic efficacy in IPF patients.
Ruan et al. reported that oral administration of fedratinib (a JAK2 inhibitor) attenuated the degree of BLM-induced pulmonary fibrosis in mice by reducing myofibroblast activation and reduced the levels of Smad3, Akt, Erk, JNK and P38 phosphorylation, suggested that fedratinib may inhibit the JAK2/STAT3 and TGF-β1/Smad signaling pathways (37). Conte et al. demonstrated that pirfenidone attenuated the TGF-β1-mediated differentiation of fibroblasts into myofibroblasts either through a canonical Smad-dependent pathway or a Smad-independent noncanonical pathway (6). We speculated that pirfenidone may inhibit Smad-independent noncanonical pathways to alleviate fibrosis. The results of a previous study suggested that nintedanib can block several signaling pathways simultaneously, for example, growth factor (TGF-β, PDGF, FGF, etc.) signaling pathways (10). Ruan et al. reported that some tyrosine kinase inhibitors, such as nintedanib, thalidomide and sunitinib, can strongly inhibit the TGF-β1/Smad3 signaling pathway, suggesting that tyrosine kinase inhibitors also have a relationship with the TGF-β1 signaling pathway (15). Chaudhary et al. also noted that many cytokines, such as TGF-β, IL-1 and TNF-α, exhibited PDGF-dependent profibrotic activity and speculated that nintedanib can interfere with JAK2 expression via targeted inhibition of TGF-β1/Smad3 in the PDGF pathway (38). Therefore, we speculated that pirfenidone and nintedanib may attenuate pulmonary fibrosis in mice via the atypical JAK2/STAT3 pathway of TGF-β1.
In this study, to evaluate the antifibrotic effects of pirfenidone and nintedanib, the lung tissues of mice in each group were HE- and Masson-stained. The results indicated that pirfenidone and nintedanib significantly reversed BLM-induced pulmonary fibrosis, a finding that is consistent with the results of previous studies (4,6). Western blots showed that after pirfenidone and nintedanib treatment, the expression of JAK2, p-JAK2, and p-STAT3 significantly decreased, and the expression of TGF-βR2, SMA (a myofibroblast activation marker), and ECM fibronectin decreased. Fluorescence microscopy showed that the expression of p-JAK2 in the lung tissues of mice in the BLM group increased and that the fluorescence was mainly localized in the nucleus. After pirfenidone and nintedanib treatment, the fluorescence signal of p-JAK2 decreased. Previously, the role of JAK in signal transduction was thought to be limited to an activator of STATs in the cytoplasm, but in recent years, p-JAK2 has also been detected in the nucleus (39,40). Montero et al. found that p-JAK2 was present at increased levels in BLM-treated rat lung tissue and was located in the nucleus of cells in fibrotic areas (16). The discovery of p-JAK2 in the nucleus means that p-JAK2 can function independently of STAT3.This experiment also revealed that p-JAK2 in mouse lung tissue was mainly localized in the nucleus. Therefore, pirfenidone and nintedanib may inhibit the expression of JAK2 by affecting the TGF-β1/TGF-βR2 signaling pathway, thus attenuating pulmonary fibrosis.
Previous study has indicated that activation of the TGF-β1 receptor and subsequent Smad signaling is not sufficient to drive EMT (38). Therefore, many other signaling pathways can also affect EMT. For example, TGF-β1 can activate some non-Smad signaling pathways (such as the MAPK, AKT, PAR6 and Shc signaling pathways) and the JAK2 signaling pathway. Dees et al. proposed that JAK2 is activated in a TGF-β-dependent manner in systemic sclerosis and that the expression of p-JAK2 and p-STAT3 is elevated under TGF-β stimulation (41). Similarly, You et al. noted that the TGF-β1 signaling pathway can induce JAK2 phosphorylation and activate the JAK2/STAT3 signaling pathway (42). Furthermore, Ruan et al. reported that fedratinib (a targeted selective JAK2 tyrosine kinase inhibitor) inhibits the inflammation and fibrosis caused by TGF-β1 and IL-6 by targeting the JAK2 receptor (37). In conclusion, there is an interaction between the TGF-β1/SMAD3 and JAK2/STAT3 signaling pathways.
We further explored the relationship between TGF-β1 and JAK2 in cell experiments and found that 50 ng/mL TGF-β1 strongly stimulated the expression of JAK2, p-JAK2, p-STAT3, and SMA in MLE12 cells. JAK2 expression was inhibited by the TGF-β1 receptor inhibitor LY2109761 in a dose-dependent manner; that is, JAK2 expression decreased greatly with increasing concentrations of LY2109761. When pirfenidone and nintedanib were used to inhibit TGF-β1 and TGF-β1 receptors, the expression of JAK2 and p-JAK2 significantly decreased, and the corresponding expression of SMA and ECM also decreased. Conversely, when JAK2 gene expression was downregulated, the expression of TGF-βR2 and SMA decreased, suggesting that downregulating the expression of JAK2 can significantly affect the activation of the TGF-β1 signaling pathway.
Conclusions
In summary, TGF-β1 promotes JAK2 phosphorylation, and by blocking the TGF-β1/TGF-βR signaling pathway inhibits JAK2 phosphorylation. This may represent one of the antifibrotic mechanisms of pirfenidone and nintedanib. Interestingly, after JAK2 expression was downregulated, TGF-βR2 and SMA expression significantly decreased. These results indicate that inhibiting the expression of JAK2 can affect the TGF-β1 signaling pathway and further inhibit the occurrence of EMT and fibrosis. It is speculated that there may be mutual influence between the JAK2 and TGF-β1 signaling pathways. These results provide new insights for the treatment of pulmonary fibrosis.
Acknowledgments
Thanks to Hongli Liu of the Chongqing Medical University for helping with the experiment.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1057/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1057/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1057/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-23-1057/coif). J.Z. was supported by the Chongqing Clinical Research Center for Geriatric Diseases; Chongqing Science and Health Joint Medical Research Project 2020GDRC012c; Young and Middle-aged Senior Medical Talents studio of Chongqing under Grant ZQNYXGDRCGZS2021007; Chongqing Entrepreneurship and Innovation Support Program for Overseas Students Returning to China under Grant cx2019102; Chongqing Clinical Research Centre for Geriatric Diseases Project (2020-126); and 2023 Chongqing Natural Science Foundation (CSTB2023NSCQ-MSX0188). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. Dossy20210119001) granted by the Ethics Committee of Chengdu Dashuo Biotechnology Co., in compliance with institutional guidelines for the care and use of animals. Since we do not have an animal breeding facility, all of our animal experiments are animal experiments done at Chengdu Dashuo Biotechnology Co.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet 2017;389:1941-52. [Crossref] [PubMed]
- Confalonieri P, Volpe MC, Jacob J, et al. Regeneration or Repair? The Role of Alveolar Epithelial Cells in the Pathogenesis of Idiopathic Pulmonary Fibrosis (IPF). Cells 2022;11:2095. [Crossref] [PubMed]
- Spagnolo P, Kropski JA, Jones MG, et al. Idiopathic pulmonary fibrosis: Disease mechanisms and drug development. Pharmacol Ther 2021;222:107798. [Crossref] [PubMed]
- Sgalla G, Iovene B, Calvello M, et al. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res 2018;19:32. [Crossref] [PubMed]
- Inomata M, Kamio K, Azuma A, et al. Pirfenidone inhibits fibrocyte accumulation in the lungs in bleomycin-induced murine pulmonary fibrosis. Respir Res 2014;15:16. [Crossref] [PubMed]
- Conte E, Gili E, Fagone E, et al. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci 2014;58:13-9. [Crossref] [PubMed]
- Liu J, Shi G. Pirfenidone activates cannabinoid receptor 2 in a mouse model of bleomycin-induced pulmonary fibrosis. Exp Ther Med 2019;18:4241-8. [Crossref] [PubMed]
- Wollin L, Distler JHW, Redente EF, et al. Potential of nintedanib in treatment of progressive fibrosing interstitial lung diseases. Eur Respir J 2019;54:1900161. [Crossref] [PubMed]
- Öztürk Akcora B, Storm G, Prakash J, et al. Tyrosine kinase inhibitor BIBF1120 ameliorates inflammation, angiogenesis and fibrosis in CCl(4)-induced liver fibrogenesis mouse model. Sci Rep 2017;7:44545. [Crossref] [PubMed]
- Sivakumar P, Kitson C, Jarai G. Modeling and measuring extracellular matrix alterations in fibrosis: challenges and perspectives for antifibrotic drug discovery. Connect Tissue Res 2019;60:62-70. [Crossref] [PubMed]
- Riesco-Martinez MC, Sanchez-Torre A, Garcia-Carbonero R. Safety and efficacy of nintedanib for the treatment of metastatic colorectal cancer. Expert Opin Investig Drugs 2017;26:1295-305. [Crossref] [PubMed]
- Okano T, Kobayashi T, Yasuma T, et al. Low-Dose of Intrapulmonary Pirfenidone Improves Human Transforming Growth Factorβ1-Driven Lung Fibrosis. Front Pharmacol 2020;11:593620. [Crossref] [PubMed]
- Kinoshita T, Goto T. Molecular Mechanisms of Pulmonary Fibrogenesis and Its Progression to Lung Cancer: A Review. Int J Mol Sci 2019;20:1461. [Crossref] [PubMed]
- Inui N, Sakai S, Kitagawa M. Molecular Pathogenesis of Pulmonary Fibrosis, with Focus on Pathways Related to TGF-β and the Ubiquitin-Proteasome Pathway. Int J Mol Sci 2021;22:6107. [Crossref] [PubMed]
- Ruan H, Lv Z, Liu S, et al. Anlotinib attenuated bleomycin-induced pulmonary fibrosis via the TGF-β1 signalling pathway. J Pharm Pharmacol 2020;72:44-55. [Crossref] [PubMed]
- Montero P, Milara J, Roger I, et al. Role of JAK/STAT in Interstitial Lung Diseases; Molecular and Cellular Mechanisms. Int J Mol Sci 2021;22:6211. [Crossref] [PubMed]
- Tieyuan Z, Ying Z, Xinghua Z, et al. Piceatannol-mediated JAK2/STAT3 signaling pathway inhibition contributes to the alleviation of oxidative injury and collagen synthesis during pulmonary fibrosis. Int Immunopharmacol 2022;111:109107. [Crossref] [PubMed]
- Milara J, Ballester B, Morell A, et al. JAK2 mediates lung fibrosis, pulmonary vascular remodelling and hypertension in idiopathic pulmonary fibrosis: an experimental study. Thorax 2018;73:519-29. [Crossref] [PubMed]
- Milara J, Hernandez G, Ballester B, et al. The JAK2 pathway is activated in idiopathic pulmonary fibrosis. Respir Res 2018;19:24. [Crossref] [PubMed]
- Kurita Y, Araya J, Minagawa S, et al. Pirfenidone inhibits myofibroblast differentiation and lung fibrosis development during insufficient mitophagy. Respir Res 2017;18:114. [Crossref] [PubMed]
- Wollin L, Maillet I, Quesniaux V, et al. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther 2014;349:209-20. [Crossref] [PubMed]
- Zhou Y, Li P, Duan JX, et al. Aucubin Alleviates Bleomycin-Induced Pulmonary Fibrosis in a Mouse Model. Inflammation 2017;40:2062-73. [Crossref] [PubMed]
- Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 2014;9:157-79. [Crossref] [PubMed]
- Xiao K, He W, Guan W, et al. Mesenchymal stem cells reverse EMT process through blocking the activation of NF-κB and Hedgehog pathways in LPS-induced acute lung injury. Cell Death Dis 2020;11:863. [Crossref] [PubMed]
- Cheng H, Feng D, Li X, et al. Iron deposition-induced ferroptosis in alveolar type II cells promotes the development of pulmonary fibrosis. Biochim Biophys Acta Mol Basis Dis 2021;1867:166204. [Crossref] [PubMed]
- Ying H, Fang M, Hang QQ, et al. Pirfenidone modulates macrophage polarization and ameliorates radiation-induced lung fibrosis by inhibiting the TGF-β1/Smad3 pathway. J Cell Mol Med 2021;25:8662-75. [Crossref] [PubMed]
- Liu T, De Los Santos FG, Phan SH. The Bleomycin Model of Pulmonary Fibrosis. Methods Mol Biol 2017;1627:27-42. [Crossref] [PubMed]
- Martins V, Teodoro WR, Velosa APP, et al. Butylated hydroxytoluene induces type-V collagen and overexpression of remodeling genes/proteins in experimental lung fibrosis. Histol Histopathol 2018;33:1111-23. [Crossref] [PubMed]
- Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;294:L152-60. [Crossref] [PubMed]
- Stainer A, Faverio P, Busnelli S, et al. Molecular Biomarkers in Idiopathic Pulmonary Fibrosis: State of the Art and Future Directions. Int J Mol Sci 2021;22:6255. [Crossref] [PubMed]
- Hanaka T, Kido T, Noguchi S, et al. The overexpression of peroxiredoxin-4 affects the progression of idiopathic pulmonary fibrosis. BMC Pulm Med 2019;19:265. [Crossref] [PubMed]
- Wei Y, Kim TJ, Peng DH, et al. Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis. J Clin Invest 2017;127:3675-88. [Crossref] [PubMed]
- Samukawa T, Hamada T, Uto H, et al. The elevation of serum napsin A in idiopathic pulmonary fibrosis, compared with KL-6, surfactant protein-A and surfactant protein-D. BMC Pulm Med 2012;12:55. [Crossref] [PubMed]
- Vij R, Noth I. Peripheral blood biomarkers in idiopathic pulmonary fibrosis. Transl Res 2012;159:218-27. [Crossref] [PubMed]
- Ikeda K, Chiba H, Nishikiori H, et al. Serum surfactant protein D as a predictive biomarker for the efficacy of pirfenidone in patients with idiopathic pulmonary fibrosis: a post-hoc analysis of the phase 3 trial in Japan. Respir Res 2020;21:316. [Crossref] [PubMed]
- Kim K, Shin D, Lee G, et al. Loss of SP-A in the Lung Exacerbates Pulmonary Fibrosis. Int J Mol Sci 2022;23:5292. [Crossref] [PubMed]
- Ruan H, Luan J, Gao S, et al. Fedratinib Attenuates Bleomycin-Induced Pulmonary Fibrosis via the JAK2/STAT3 and TGF-β1 Signaling Pathway. Molecules 2021;26:4491. [Crossref] [PubMed]
- Chaudhary NI, Roth GJ, Hilberg F, et al. Inhibition of PDGF, VEGF and FGF signalling attenuates fibrosis. Eur Respir J 2007;29:976-85. [Crossref] [PubMed]
- Zouein FA, Duhé RJ, Booz GW. JAKs go nuclear: emerging role of nuclear JAK1 and JAK2 in gene expression and cell growth. Growth Factors 2011;29:245-52. [Crossref] [PubMed]
- Qian CJ, Yao J, Si JM. Nuclear JAK2: form and function in cancer. Anat Rec (Hoboken) 2011;294:1446-59. [Crossref] [PubMed]
- Dees C, Tomcik M, Palumbo-Zerr K, et al. JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor β in systemic sclerosis. Arthritis Rheum 2012;64:3006-15. [Crossref] [PubMed]
- You X, Jiang X, Zhang C, et al. Dihydroartemisinin attenuates pulmonary inflammation and fibrosis in rats by suppressing JAK2/STAT3 signaling. Aging (Albany NY) 2022;14:1110-27. [Crossref] [PubMed]


