Post-implantation syndrome after frozen elephant trunk is associated with the volume of new-onset aortic thrombus
Introduction
In the last decades, the frozen elephant trunk (FET) technique has gained increasing popularity for the treatment of patients with complex thoracoabdominal aortic pathologies involving the aortic arch and the descending aorta (1). The peculiarity of this hybrid technique lies in its combination of an open and an endovascular approach ensuring reduced operative trauma when compared to extensive thoracoabdominal open aortic surgery (2). The use of a hybrid prosthesis consisting of a distal stent-bearing segment and of a branched vascular tube for the reconstruction of the aortic arch allows a staged aortic repair in the case of extensive aortic disease, and reduces the risk of intra- and perioperative complications (3).
Post-implantation syndrome (PIS) is a well-known clinical entity after endovascular repair. It was first described by Velazquez et al. (4) and is defined as continuous fever despite antimicrobial therapy with a concomitant rise of inflammatory markers shortly after endovascular aortic repair (EVAR). PIS has been observed in nearly one-third of EVAR patients and is believed to be transient and harmless in most cases. The length of stent graft implanted, its composition, as well as the volume of new-onset mural aortic thrombus have been identified as possible risk factors promoting the occurrence of PIS (5,6). The exact pathophysiology of PIS and its effects on long-term clinical outcomes remain unclear. Furthermore, its effect on patient outcomes is a concern because it may lead to prolonged hospitalisation (7-10).
PIS as a clinical entity after using the FET technique has not been clearly investigated. The current study aims to define its incidence after using the FET in patients undergoing extensive aortic replacement, and to identify possible triggering factors. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-398).
Methods
Study design and patient population
This was a single-centre, retrospective, observational study on a total of 59 consecutive patients who underwent aortic replacement using the FET technique between February 2015 and April 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the local ethics committee (Ethik-Kommission Muenster, protocol number 2018-506-f-S) and individual consent for this retrospective analysis was waived.
Patient demographics, comorbidities, intraoperative data as well as pre- and postoperative computed tomographic angiogram (CTA) findings (maximum aortic diameter, volume of mural thrombus) and vital parameters were analysed. Body temperature was recorded every eight hours during the entire duration of hospitalisation. Bladder temperature was preferentially used for the data analysis. In cases where bladder temperature measurements were unavailable, tympanic measurement was used. White blood cell count (WBCC), procalcitonin (PCT) and serum C-reactive protein (CRP) levels were assessed serially one day before surgery and daily during the first seven postoperative days. The results of the microbiological cultures obtained during the postoperative period were also analysed.
Exclusion criteria
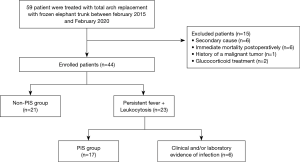
Patients on treatment with glucocorticoid or immunosuppressive drugs at the time of treatment and patients affected by malignant tumour, pre-existing traumatic injuries (major trauma or other surgery during the two weeks before) were excluded from the study. Furthermore, patients who died during the first postoperative week, patients who underwent early revision as well as patients with a diagnosed infection focus were excluded from the study cohort (Figure 1).
The FET technique
The FET technique has been described early (11). A prophylactic antibiotic (Cefazolin, 2 g) was routinely administered to all patients before and after the procedure (for 24 hours). Surgery was performed under moderate hypothermic (28 °C) circulatory arrest and selective bilateral antegrade cerebral perfusion. Near-infrared spectroscopy was used to monitor cerebral tissue oxygenation. After median sternotomy, an antegrade extracorporeal circulation connection via cannulation of the right subclavian artery was initiated. Myocardial protection was achieved using retrograde blood cardioplaegia. After distal transection of the aortic arch the Thoraflex™ Hybrid Plexus Prosthesis (Vascutek, Terumo Aortic, Inchinnan, Scotland) was positioned in the descending aorta. The stent-graft size was determined according to the maximal diameter of the lumen of the descending aorta on preoperative CT scans. After completion of the distal anastomosis in zone 3, aortic arch vessel reconstruction was performed under reinitialised perfusion of the lower body. All patients were treated by the same cardiac surgeon.
PIS definition
PIS was defined as follows: (I) the presence of fever during hospitalisation defined as body temperature higher than 38 °C lasting over one day during hospitalisation, with onset in the first 24 hours after surgery, and (II) leucocytosis (WBCC >12,000/µL) (6).
Thrombus volume measurement
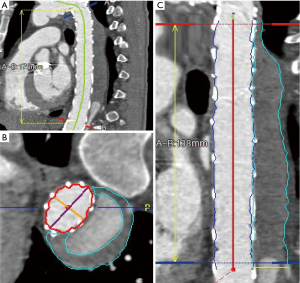
Pre- and postoperative ECG-gated computed tomography angiograms (CTAs) were used to perform thrombus volumetric measurements. First, a multiplanar reconstruction of the CT angiograms was performed with the use of the Aquarius iNtuition software (TeraRecon Inc, Foster City, CA, USA). Second, after curved planar reformation, a centreline was automatically generated and manually corrected in the true lumen and then after selection of the preset function for volume measuring, the false lumen and true lumen were divided automatically from the software by detecting the level of contrast medium enhancement. The area of thrombus material and true lumen volumes were measured manually and separately from the left subclavian artery to the celiac trunk in each CT slice. In the end, the volume of thrombus material (cubic centimetres) was measured automatically due to the addition of all marked thrombus areas together. Preoperative chronic mural thrombus and the postoperative total volume of thrombus were measured in the venous phase. The new-onset thrombus volume was obtained by subtracting the preoperative chronic mural thrombus measured volume from the postoperative total thrombus measured volume (11). Previous volume rendering methods are shown in Figure 2.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation for parametric data and median with interquartile range for non-parametric data, whereas dichotomous variables are presented as crude numbers and percentages. Comparisons of continuous variables were performed using a Student t test for normally distributed variables, and a Mann-Whitney U test for non-normally distributed variables. A chi-square test or Fisher`s exact test was used for categorical variables. In addition, correlations between the measured variables in each group were analysed using Pearson’s correlation method. A multivariate regression analysis with significant variables was performed. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics for Windows version 26.0 (IBM Corp., Armonk, NY, USA).
Results
During the study period, 59 patients underwent a FET procedure. Based on the study recruitment criteria, 21 patients were excluded due to chronic glucocorticoid therapy (n=2), known malignant tumour (n=1), secondary cause (n=6) postoperative bleeding, reoperation or revision (n=6), or fever of infectious aetiology (n=6). Thirty-eight patients (58.5±10.2 years, 68.5% men) were included in the study. Among these 38 patients, 17 (44.7%) met the criteria for postoperative PIS (Figure 1).
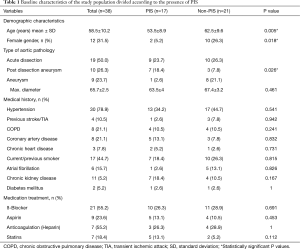
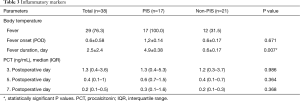
Patients with PIS were significantly younger than those without PIS (53.5±8.9 years vs. 62.5±9.6 years; P=0.005). Interestingly, female patients were less likely to develop PIS (5.2% vs. 26.3%, P=0.018). There were no significant differences between the PIS and non-PIS groups regarding the incidence of known atherosclerotic risk factors, as well as chronic kidney disease and smoking status. Furthermore, patients treated due to a chronic aortic dissection (post-dissection aneurysm) developed PIS significantly more often than those who were treated due to an isolated aortic aneurysm (P=0.026). There were no significant differences between the two groups in terms of acute aortic dissection (Table 1).
Full table
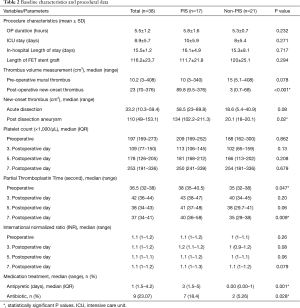
The length of the procedure, ICU stay and in-hospital stay at our institution were longer for patients who developed PIS; however, there was no statistically significant difference between the two groups (Table 2). CTA was performed for all patients before discharge, after a mean time of 4.3±4.0 days.
Full table
For the thrombus volume, 62 measurements were obtained starting from the left subclavian artery and ending just proximally to the celiac trunk. Twenty-eight patient CTAs were evaluated for acute dissection, 18 for chronic dissection/post-dissection aneurysm and 16 for non-dissection aneurysm. The volume of new-onset thrombus was significantly higher in patients with PIS (P<0.001), whereas no statistically significant difference was found in the volume of preoperative chronic mural thrombus between patients with and without PIS (Table 2). In addition, patients treated for chronic aortic dissection (post-dissection aneurysm) had, postoperatively, significantly more thrombus material developed in the false lumen (P=0.02). The partial thromboplastin time (PTT) value was significantly higher in the PIS group preoperatively and on the seventh postoperative day. No statistically significant differences between the PIS and non-PIS groups in terms of coagulation factors were detected (Tables 1 and 2). Patients in the PIS group received significantly more antibiotics (P=0.028) and antipyretics (P=0.001) (Table 2).
On the first postoperative day, 12 patients in the non-PIS group developed a body temperature higher than 38 °C, lasting less than 24 hours. The duration of postoperative fever was significantly higher in the PIS group than in the non-PIS group (4.9±0.38 vs. 0.6±0.17 days, P=0.007), but there were no significant differences in the time interval between the index procedure and the onset of fever between the groups (Table 3). On the operation day and first postoperative day, body temperature, WBCC and CRP were not significantly different between the two groups. The temperature increased in the PIS group rapidly and remained significantly higher over the next seven days after operation (Figure 3).
Full table
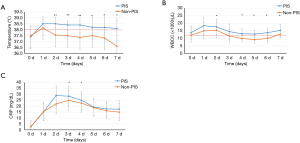
The development of leucocytosis in the PIS group peaked rapidly within 24 hours, while CRP levels peaked in the first 48 hours after operation. CRP concentration was significantly higher in the PIS group on the third and fourth postoperative days than in the non-PIS group, while the WBCC was significantly higher in the PIS group on the second and from the fourth to the seventh postoperative days than in the non-PIS group (Figure 3). Procalcitonin was not significantly different between the two groups (Table 3).
The median volume of mural thrombus detected on the preoperative CTAs was 10.2 cm3 (3–408) while the median volume of new-onset thrombus after the procedure was 23 cm3 (70–376). A statistically significant correlation was found between the volume of new-onset thrombus and CRP level on the third postoperative day (POD) (r=0.531; P=0.003), as well as duration of fever (r=0.618; P<0.001), the increase in WBCC on the fifth POD (r=0.432; P=0.019) and on the seventh POD (r=0.456; P<0.017). After multivariate analysis, only the correlation between the volume of new-onset thrombus remained independently associated with the CRP level on the third POD (β =0.488; P=0.011) (Table 4).

Full table
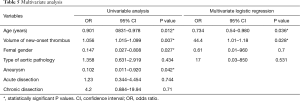
The following variables, which significantly differed between the two study groups, were addressed in a univariable analysis: age, gender, type of aortic pathology, volume of new-onset thrombus. In the multivariate logistic regression, the volume of new-onset thrombus (P=0.028) and age (P=0.036) remained the variables associated with a statistically significant increased incidence of PIS (Table 5).
Full table
Discussion
In the current study a positive association between increased volume of new-onset thrombus and the occurrence of PIS in patients undergoing the FET was detected.
Postoperative fever after cardiac surgery can result from an infectious or non-infectious aetiology. Previously described causes of non-infectious postoperative fever include surgical trauma, cardiopulmonary bypass, bleeding, transfusion reaction and drug reactions (12). PIS as a source of fever of non-infectious aetiology after the FET has not yet been investigated. To identify patients with PIS could reduce the possible empirical use of antibiotics and of invasive imaging in order to rule out possible infectious processes.
The definition of PIS is variable in the current literature (6,13-16). In the remaining cohort, the occurrence of PIS was hypothesised by the presence of fever (>38 °C lasting longer than one day) and leukocytosis (white blood cell count >12,000/µL). In the present study, a substantial quote of the patients met the criteria for postoperative PIS, with an estimated incidence of 44.7% (n=17). In the literature referring to endovascular procedures, PIS incidence varies from 15.8% to 34% after TEVAR (thoracic endovascular aortic repair) and from 14% to 60% after EVAR. This variability is due to the different diagnostic criteria and definitions used (6,13-16). Some authors use as criteria the development of fever and CRP elevation using the cut-off value of 10 mg/dL, while most use as criteria the occurrence of fever (>38 °C) and leucocytosis (>12,000/µL) (5,6,17-20). In our study, the CRP levels remained higher than 10 mg/dl within the first five days after a major operation such as sternotomy and aortic arch replacement using the FET technique. PIS was defined by the presence of leucocytosis (>12,000/µL) and fever (>38 °C) lasting longer than one day, with onset in the first 24 hours after surgery; patients with an infection focus or at risk for non-infectious fever (postoperative bleeding, reoperation or revision after the FET) were excluded.
The pathophysiology underlying PIS is not well understood and the relation of this systemic inflammatory response with patient outcomes has not been established. PIS causes prolonged hospitalisation and difficulty in postoperative recovery, although most of the cases are known to be benign and self-limiting (9,15-17). In our study, we could not show any significant difference in length of stay between PIS and non-PIS. This may be the result of our policy of transferring treated patients early to rehabilitation clinics. Several studies have reported that a serious systemic inflammatory response after EVAR might result in a lower quality of life (21). Nano et al. (21) reported that patients with postoperative PIS felt significantly more limited in performing their daily physical activities and were more emotionally discouraged and depressed about their state of health than the group that did not develop PIS.
There are very few studies investigating the correlation between the occurrence of PIS and aortic thrombus volume. Kakisis et al. (7) reported that both the volume of new-onset thrombus and the type of graft were independently associated with the development of PIS in patients undergoing elective EVAR. However, no significant relationship was observed with the volume of chronic mural thrombus.
The association between increased inflammation and thrombus volume reported by Kakisis et al. (7) and Lee et al. (20) supports the possible role for sac thrombus in PIS. The mechanisms behind the thrombosis of the false lumen in the case of aortic dissections are varied. The so-called “aortic remodelling” consequent to the endovascular exclusion of the false lumen has been seen as the most relevant factor triggering the occurrence and extension of postoperative thrombosis (22-25). However, the extension of concomitant atherosclerotic lesions, the changes of local haemodynamics inside the aorta, as well as the activation of or blood coagulation and haemolysis can be seen as concurrent factors in activating the thrombotic reaction after the FET and endovascular treatment in general (26,27). In the current study, there were no statistically significant differences in preoperative mural thrombus volumes between PIS and non-PIS patients. The volume of new-onset thrombus remained the only variable associated with a statistically significant increased incidence of PIS and increased CRP level on the third postoperative day.
The stent-graft construction material appears to be one of the major risk factors for the development of PIS (8,16,17,28). The Thoraflex™ Hybrid prosthesis consists of a proximal unstented sealed woven PET (polyethylenterephthalat) tube with four integrated side branches and a distal stented part made of polyester and nitinol stents. The possible effects of different graft types on the occurrence of PIS, postoperative fever and on the increase of inflammatory markers have been widely investigated. Most of the studies report a more frequent occurrence of PIS in the case of stent grafts made of woven polyester than in those made of ePTFE (8,16,17,28). Arnaoutoglou et al. (6) found that the use of a polyester endograft independently predicted PIS and was correlated with an above 10 times higher risk for an inflammatory response.
In the present study, the age of patients was an independent predictor for PIS. Ker et al. (29) agree with this concept of PIS as an immune reaction. As younger patients have stronger immune reactions, more invasive strategies and a longer aortic conversion can trigger a more extensive immune reaction in these patients.
The current study showed that patients treated for chronic aortic dissection (post-dissection aneurysm) developed significantly more thrombus material in the false lumen postoperatively compared to patients treated for acute dissection. This could be related to remodelling of the aorta after the Thoraflex hybrid prosthesis implantation in the acute dissection group. This is supported by recently published data demonstrating significant volume changes in true lumen in the descending aorta after FET procedure using the Thoraflex hybrid prosthesis (11). We were able to show that use of the FET procedure for acute aortic dissection leads to a statistically measurable increase in the true lumen volume from the left subclavian artery to the celiac trunk at the short-term follow-up examination (upon discharge) (11).
Limitations
Among the limitations of the current study, its retrospective character as well as its small sample size should be mentioned. Furthermore, the etherogenity of the pathologies treated (aortic aneurysm and acute/chronic aortic dissections) may have influenced the occurrence of PIS. The retrospective design of the study did not allow us to investigate the presence of other circulating inflammatory markers such as interleukins and tumour necrosis factors, in order to identify possible triggering pathways of PIS. Furthermore, even though the two study groups did not differ in terms of occurrence of concomitant pathologies such as chronic kidney disease, active smoking and diabetes mellitus, the possible influence of these proinflammatory factors in determining a postoperative inflammatory reaction cannot be completely excluded.
Conclusions
PIS can trigger non-infectious fever after the FET. In the present study, the volume of new-onset aortic thrombus was associated with the development of PIS. Prospective studies on larger sample sizes possibly investigating the use of different prostheses could lead to a better understanding of the mechanisms, physiology and risk factors of PIS after the FET.
Acknowledgments
The manuscript was revised by a native English speaker (Anchor English, Inchnacardoch, Fort Augustus, Inverness-shire, UK, PH32 4BN).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-398
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-398
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-398
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-398). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the local ethics committee (Ethik-Kommission Muenster, protocol number 2018-506-f-S) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Takagi H, Umemoto T. ALICE Group. A Meta-Analysis of Total Arch Replacement With Frozen Elephant Trunk in Acute Type A Aortic Dissection. Vasc Endovascular Surg 2016;50:33-46. [Crossref] [PubMed]
- Jakob H, Tsagakis K, Tossios P, et al. Combining classic surgery with descending stent grafting for acute DeBakey type I dissection. Ann Thorac Surg 2008;86:95-101. [Crossref] [PubMed]
- Di Eusanio M, Borger M, Petridis FD, et al. Conventional versus frozen elephant trunk surgery for extensive disease of the thoracic aorta. J Cardiovasc Med (Hagerstown) 2014;15:803-9. [Crossref] [PubMed]
- Velázquez OC, Carpenter JP, Baum RA, et al. Perigraft air, fever, and leukocytosis after endovascular repair of abdominal aortic aneurysms. Am J Surg 1999;178:185-9. [Crossref] [PubMed]
- Baek JK, Kwon H, Ko GY, et al. Impact of graft composition on the systemic inflammatory response after an elective repair of an abdominal aortic aneurysm. Ann Surg Treat Res 2015;88:21-7. [Crossref] [PubMed]
- Arnaoutoglou E, Kouvelos G, Papa N, et al. Prospective evaluation of post-implantation inflammatory response after EVAR for AAA: influence on patients' 30 day outcome. Eur J Vasc Endovasc Surg 2015;49:175-83. [Crossref] [PubMed]
- Kakisis JD, Moulakakis KG, Antonopoulos CN, et al. Volume of new-onset thrombus is associated with the development of postimplantation syndrome after endovascular aneurysm repair. J Vasc Surg 2014;60:1140-5. [Crossref] [PubMed]
- Zhu Y, Luo S, Ding H, et al. Predictors associated with an increased prevalence of postimplantation syndrome after thoracic endovascular aortic repair for type B aortic dissection†. Eur J Cardiothorac Surg 2019;55:998-1005. [Crossref] [PubMed]
- Arnaoutoglou E, Kouvelos G, Milionis H, et al. Post-implantation syndrome following endovascular abdominal aortic aneurysm repair: preliminary data. Interact Cardiovasc Thorac Surg 2011;12:609-14. [Crossref] [PubMed]
- Moulakakis KG, Alepaki M, Sfyroeras GS, et al. The impact of endograft type on inflammatory response after endovascular treatment of abdominal aortic aneurysm. J Vasc Surg 2013;57:668-77. [Crossref] [PubMed]
- Usai MV, Ibrahim A, Oberhuber A, et al. Quantification of volume changes in the descending aorta after frozen elephant trunk procedure using the Thoraflex hybrid prosthesis for type A aortic dissection. J Thorac Dis 2021;13:60-6. [Crossref] [PubMed]
- Rhee C, Sax PE. Evaluation of fever and infections in cardiac surgery patients. Semin Cardiothorac Vasc Anesth 2015;19:143-53. [Crossref] [PubMed]
- Gorla R, Erbel R, Kahlert P, et al. Clinical features and prognostic value of stent-graft-induced post-implantation syndrome after thoracic endovascular aortic repair in patients with type B acute aortic syndromes. Eur J Cardiothorac Surg 2016;49:1239-47. [Crossref] [PubMed]
- Wu M, Zhang L, Bao J, et al. Postoperative glucocorticoid enhances recovery after endovascular aortic repair for chronic type B aortic dissection: a single-center experience. BMC Cardiovasc Disord 2016;16:59. [Crossref] [PubMed]
- de la Motte L, Kehlet H, Vogt K, et al. Preoperative methylprednisolone enhances recovery after endovascular aortic repair: a randomized, double-blind, placebo-controlled clinical trial. Ann Surg 2014;260:540-8; discussion 548-9. [Crossref] [PubMed]
- Ferreira F, Machado R, Martins J, et al. Post-implantation syndrome – retrospective analysis of 52 patients. Angiologia e Cirurgia Vascular 2015;11:204-8. [Crossref]
- Voûte MT, Bastos Gonçalves FM, van de Luijtgaarden KM, et al. Stent graft composition plays a material role in the postimplantation syndrome. J Vasc Surg 2012;56:1503-9. [Crossref] [PubMed]
- Kwon H, Ko GY, Kim MJ, et al. Effects of postimplantation systemic inflammatory response on long-term clinical outcomes after endovascular aneurysm repair of an abdominal aortic aneurysm. Medicine (Baltimore) 2016;95:e4532 [Crossref] [PubMed]
- Sartipy F, Lindström D, Gillgren P, et al. The role of procalcitonin in postimplantation syndrome after EVAR: a pilot study. Ann Vasc Surg 2014;28:866-73. [Crossref] [PubMed]
- Lee JH, Choi JH, Kim EJ. Volume of mural thrombus plays a role in the elevation of inflammatory markers after endovascular aortic repair. J Cardiothorac Surg 2018;13:27. [Crossref] [PubMed]
- Nano G, Occhiuto MT, Stegher S, et al. Postimplantation syndrome after endovascular aortic repair using the Anaconda™ endograft. Ann Vasc Surg 2014;28:1409-15. [Crossref] [PubMed]
- Weiss G, Santer D, Dumfarth J, et al. Evaluation of the downstream aorta after frozen elephant trunk repair for aortic dissections in terms of diameter and false lumen status. Eur J Cardiothorac Surg 2016;49:118-24. [Crossref] [PubMed]
- Haensig M, Schmidt A, Staab H, et al. Endovascular Repair of the Thoracic or Thoracoabdominal Aorta Following the Frozen Elephant Trunk Procedure. Ann Thorac Surg 2020;109:695-701. [Crossref] [PubMed]
- Kozlov BN, Panfilov DS, Saushkin VV, et al. Distal aortic remodelling after the standard and the elongated frozen elephant trunk procedure. Interact Cardiovasc Thorac Surg 2019;29:117-23. [Crossref] [PubMed]
- Iafrancesco M, Goebel N, Mascaro J, et al. Aortic diameter remodelling after the frozen elephant trunk technique in aortic dissection: results from an international multicentre registry. Eur J Cardiothorac Surg 2017;52:310-8. [Crossref] [PubMed]
- Shimazaki T, Ishimaru S, Kawaguchi S, et al. Blood coagulation and fibrinolytic response after endovascular stent grafting of thoracic aorta. J Vasc Surg 2003;37:1213-8. [Crossref] [PubMed]
- Ma WG, Zheng J, Sun LZ, et al. Open Stented Grafts for Frozen Elephant Trunk Technique: Technical Aspects and Current Outcomes. Aorta (Stamford) 2015;3:122-35. [Crossref] [PubMed]
- Ito E, Toya N, Fukushima S, et al. Polyester Grafts Are a Risk Factor for Postimplantation Syndrome after Abdominal Endovascular Aneurysm Repair: Retrospective Analysis for Polyester Graft, Excluder®, and Endologix Powerlink®/AFX®. Ann Vasc Dis 2018;11:520-4. [Crossref] [PubMed]
- Kapetanios DM, Karkos CD, Papazoglou KO. Changes in circulating markers of coagulation and fibrinolysis after EVAR. Int Angiol 2018;37:444-50. [Crossref] [PubMed]