Thoracoscopic management of early stages of empyema: is this the golden standard?
Introduction
Pleural infection, including complicated pleural effusion or empyema, is a well-known disease that significantly increases the morbidity and mortality associated with pneumonia, with a mortality rate reaching 20% in adults. The incidence of pneumonia and para-pneumonic effusions appears to be increasing globally across all age groups and epidemiologic studies suggest that the incidence of empyema seems to have markedly increased in the last 2 decades (1). More than five decades ago, the American thoracic society described three stages in the natural course of empyema: the exudative (stage I), fibrino-purulent (stage II), and organizing phases (stage III). Diagnosis of these stages is mainly based on radiological criteria particularly computed tomography (CT) of the chest. It is best to regard these stages as a continuum and that the development of empyema associated with pneumonia is a progressive process that will start as a simple exudation and develop into the organizing fibrotic phase (2).
There are a number of treatment modalities available for treatment of empyema ranging from antibiotic treatment, instillation of intra-pleural fibrinolytics to drainage with radiologically placed pigtail catheters or a tube thoracostomy. These modalities are particularly for patients with early stage empyema and are mainly managed by chest physicians in the pulmonology department. For more advanced stages, surgical intervention via the thoracic surgeons can be performed using minimally invasive techniques including video-assisted thoracic surgery (VATS) or an open thoracotomy (3).
In a large number of centres, the recommended surgical interventions are still preserved for remnant loculated pleural effusions after the use of antibiotics or in stage III empyema; the organizing phase. Recently, VATS pleural adhesiolysis, debridement, irrigation and decortication have also been proven to serve as an effective treatment modality in the early stages of empyema, especially during the stage II fibrino-purulent stage. With more consideration of the advantages of VATS, including less pain, lower postoperative morbidities, complications, and mortalities, it has been claimed that is a reasonable initial approach for all cases of early empyema and with trials of treating the late fibrous organizing phase of empyema (4).
The aim of this study is to assess the safety and effectiveness of using upfront VATS drainage, wash, debridement and decortication by the thoracic surgeons for all surgically fit patients with early stages of empyema (stage I and II) in a tertiary referral centre. We compared this to management of early stage of empyema (stage I) by non-surgical modalities via the pulmonologists.
Methods
A prospective review of empyema patients managed in the largest two centres with highest flow of patients nationally. The study period started from December 2013 till March 2016. Two groups of patients were observed. The first group (group A) included patients with early exudative phase empyema stage I managed without surgical intervention by the chest physicians in the pulmonology department and the second group (group B) included patients with stage I (exudative) and stage II empyema (fibrino-purulent) referred for surgical management by VATS in the thoracic surgery department. Four thoracic surgeons and two pulmonologists were involved in the study.
Inclusion and exclusion criteria
In group A, patients presenting with chemically proven exudative stage I empyema of any amount. Patients treated with all non-surgical modalities were included. This included appropriate antibiotic therapy, drainage (pigtail/thoracostomy tube) and intra-pleural instillation of fibrinolytics. Patient who were referred for surgery were also included.
In group B, patients were referred for surgery in stage I empyema (upon physician opinion of a more surgical benefit) or stage II fibrino-purulent phase. All patients received an upfront VATS procedure regardless of any previous treatment similar to group A. All patients were on appropriate antibiotics. Patients who had intraoperative conversion to an open procedure were included. Patients not fit for a general anesthetic and those who had an upfront open decortication were excluded.
Operative technique for group B
All patients were operated using double lumen endotracheal intubation. A single port technique was utilised. Adequate drainage was performed. Debridement, adhesiolysis and irrigation with warm saline followed. A partial decortication was performed if felt to be necessary and adequate lung expansion was assured at the end of the procedure.
For both groups A and B
All patient demographics, preadmission co-morbidities, onset of chest symptoms to intervention, cause and stage of empyema, white cell count, hospital course, in hospital morbidities and mortality were recorded. A follow up period of at least 6 months was performed and the need for further intervention was recorded.
All patients were consented for operative procedure and the enrollment in the study. A new study application was permitted from the department of research in Ain Shams University (ASU-RG2-2013-17). An institutional research board approval (IRB) for the study was obtained.
Statistics
All data were entered into an Excel spreadsheet (Microsoft, Bellevue, WA, USA). Data were analyzed using IBM SPSS Statistics Version 20 (IBM SPSS Software, Armonk, NY, USA). Data analysis included analysis of variance, Chi-square test, and Fisher exact test for discrete variables. Data are reported as the mean/range. A P value less than 0.05 was considered statistically significant.
Results
A total of 66 patients were included in the study. Group A which was managed by the chest physicians comprised 28 patients while group B managed by the surgeons comprised 38 patients.
Pre-intervention characteristics
Group A
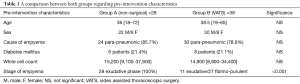
Twenty-eight patients had non-surgical management for empyema. There were 22 males and 6 females. Twenty-four patients (85.7%) had post-pneumonic empyema. The mean age was 36 [18–72]. Onset of chest symptoms to start of treatment mean was 4 days [2–13]. All patients were in stage I exudative phase. Twenty-three patients (82.1%) had drainage (pigtail/thoracostomy) and 2 (7.1%) had intra-pleural fibrinolytics. There were 6 diabetic patients (21.4%). Mean white cell count was 15,200 [9,100–37,800].
Group B
Thirty-eight patients had a VATS procedure for empyema. There were 30 males and 8 females. Thirty patients (78.9%) had post-pneumonic empyema while 8 had post-traumatic infected haemothorax. The mean age was 38.5 [19–65]. Onset of chest symptoms to procedure duration mean was 5.5 days [2–26]. Twenty-seven patients (71.1%) were diagnosed as having stage II fibrino-purulent empyema while 11 (28.9%) has stage I exudative phase. There were 8 diabetic patients (21.1%). Mean white cell count was 14,900 [8,900–34,400].
A comparison between both groups regarding pre-intervention characteristics in shown in Table 1.
Full table
Outcome
Group A
Mean hospital stay was 22 days [7–131], mean hospital cost per patient was 7,350$ (1,900$–37,770$), 3 patients (10.7%) suffered from major morbidity (pulmonary embolism, renal failure and cerebrovascular stroke. Two patients (7.1%) died. One from sepsis and the other from multi-system organ failure. During a mean follow up period of 8 months [6–14], 4 patients (14.3%) required an open decortication via thoracotomy.
Group B
There were no operative conversions to an open procedure in any patient. Mean operative time was 46.5 minutes [24–152]. Only four patients (10.5%) required a 24-hour postoperative stay in the Intensive care unit while 34 were discharged to the ward. Mean in-hospital stay was 4.1 days [2–14]. Mean hospital cost per patient was 3,100$ (1,400$–15,630$). There was no mortality and no patient suffered from any major morbidity but 2 patients suffered from superficial wound infection and one patient from lung entrapment. During a mean follow up period of 8 months [6–14], two patients (5.3%) required reoperation and a decortication via a thoracotomy at 8 and 12 weeks after the VATS procedure. Both patients had been referred late at 21 and 24 days from onset of symptoms but were given a trial of thoracoscopic drainage.
A comparison between both groups regarding outcome in shown in Table 2.

Full table
There was no statistical difference between admission patient characteristics (sex, age, diabetes, cause of empyema and white cell count; P>0.05) apart from the stage of empyema (P<0.001) where larger proportion of patients in the surgical group were in stage II empyema which is an indication for surgery.
In regards to outcome difference between both groups there was statistically significant difference in hospital stay (P=0.004, 95% CI: 10.3–25.5), total cost per patient (P<0.001), morbidity related to treatment (P=0.039) and the need for a decortication during the follow up period (P=0.047) and all were in favour of the group B who had the VATS procedure. Mortality difference did not reach statistical significance (7% vs. 0%, P=0.094).
Discussion
Empyema is a worldwide problem that affects all age groups and carries a significant rate of mortality particularly in older age groups and patients with multiple co-morbidities, with post pneumonic infected pleural effusion remaining as the most common cause (2,3). Classically, empyema was classified as a medical problem treated by chest physicians conservatively (occasionally with additional drainage) until a diagnosis of failure of medical treatment, complication by a fistula/lung abscess, loculated empyema or progression to the fibrotic phase develop and then patients were referred to thoracic surgeons for consideration of surgery.
The duration of conservative management prior to surgical intervention is probably the most important factor in determining the success of VATS for empyema management or conversely the need to proceed with a formal decortication via a thoracotomy. This is explained by the fact of increased thickening of the visceral pleura involved in the infection process making a thoracoscopic debridement more difficult. Optimal timing of thoracoscopic intervention is suggested to be in the first 4 weeks after the onset of symptoms (5). We believe that the shorter the duration after the start of the disease process, the better the chances of success with VATS.
In a study by Bagheri and his colleges (6), the authors randomly assigned patients to antibiotic therapy/irrigation or VATS and debridement after two weeks of antibiotic therapy. They had a 40% decortication via thoracotomy rate in the non-surgical group and 10% rate in the VATS group. In another study by Cassina et al. (7), they performed VATS drainage and debridement for non-tuberculous stage II empyema in 45 patients after a mean preoperative conservative management period of 37 days. Eight of their patients (18%) needed a decortication via thoracotomy. We believe that fewer patients may have needed a formal decortication in these studies if a more upfront earlier VATS approach was adopted as in our series. Nevertheless, both patients in our surgical group who needed a decortication on follow up were among the latest in being referred after 21 and 24 days from onset of symptoms.
In assessing patients with pleural empyema, a number of factors can determine the likelihood of benefit from a thoracoscopic intervention. Patients’ performance status and fitness for surgery is crucial. Staging of empyema is assessed primarily from CT of the chest. Stage II and III empyema need surgical intervention. Patients with high volume stage I empyema (occupying more than 50% of the hemi-thorax) are more likely to have higher volume of fibrin and necrotic tissue and should be considered for a VATS approach (8). Culture of gram negative organisms is also associated with a higher chance of failure of medical treatment and the need for surgery (9). Chemical analysis of the pleural fluid with pH below 7.2, glucose less than 60 and LDH more than 3 tomes the upper of the serum level are all factors suggesting that an invasive procedure will be necessary for resolution (8). Another study has found that young age (<50.5 years) and shorter period of the chief compliant (<4.5 days) are determinant factors of failure of medical treatment in pleural infection and patients usually progress to surgical intervention (10).
The benefits of VATS in managing stage II empyema are well established. The presence of fibrous bands hinders proper drainage of loculated pockets via a conventional chest drain. Converting the pleural space into one pocket using a thoracoscope is an essential part of treatment in this group. Debridement and proper lung expansion is optimally confirmed under vision via VATS. The use of pulsed irrigation lavage when performing VATS has been described as assisting in debridement and decortication in early stages of empyema (11).
The virtues of VATS in stage I exudative phase of empyema is still debatable. We argue that even with stage I moderate to severe empyema appearing as a single pocket on a CT-chest, there is a considerable amount of necrotic tissue and debris which cannot be adequately drained via a chest drain. Clearing this material via a thoracoscope may accelerate the healing process, prevent formation of a fibrous peel necessitating later decortication and allows better expansion of the lung under thoracoscopic vision.
Our study supports the results of previous studies in showing the advantageous role of using thoracoscopy in treatment of empyema. In a randomized trial performed by Bilgin and his colleagues (12), they randomized empyema patients to receive VATS or conservative measures. They found a reduction in hospital stay, cost and the need for a thoracotomy in the VATS group. The only drawback in their study was a mixture of stage I and II empyema in the inclusion criteria.
The rate of conversion to open thoracotomy and decortication during the thoracoscopy procedure has varied between studies from 8.2% to 17.1% (6,12,13). We believe our rate of conversion was nil because of performing most procedures at an early stage and acceptance of a small pneumothorax at the end of the procedure which usually self resolves with the healing process as the lung compliance improves. Thoracoscopy for VATS has also proven to improve in-hospital mortality and improve short term survival (14).
The role of intra-pleural fibrinolytics in management of early stage empyema is still not clear. Clinical trials in adults have failed to demonstrate consistent clinical benefit with administration of intra-pleural fibrinolytics. A Cochrane review (15) of intra-pleural fibrinolytic therapy performed in 2008 that was largely based on the results of MIST1 trial, did not find a consistent benefit for using these agents. The subsequent MIST2 (16) trial was smaller in numbers and included four possible treatment options, one of which was intra-pleural administration of tPA. Again, the results failed to demonstrate a clinical benefit with the use of intra-pleural fibrinolytics. Another argument against using intra-pleural fibrinolytics was that proposed dosing regimens for fibrinolytics do not ensure effective intra-pleural fibrinolysis (17).
The consequent recommendation from a number of studies was that an alternative approach, surgical drainage by VATS, provides a more effective drainage of the pleural space with improved clinical outcomes (15,17).
Although our study emphasis the positive role of VATS in management of early stages of empyema in the adult population, there is evidence that this approach is also valid in the pediatric population. Scarci and his colleges (18) have found that early VATS intervention for children with empyema was associated with a lower aggregate in-hospital mortality rate, re-intervention rate, length of hospital stay, duration of tube thoracostomy inserted and duration of antibiotic therapy compared with patients who underwent non-operative therapy.
One of main aims of thoracoscopic treatment of early stages of empyema is to halt the progress of the disease to its fibrotic stage III phase. This is achieved by allowing good drainage of pus, debridement and removal of all necrotic tissue and achieving an adequate visualized lung expansion. Nevertheless, patients who progress to the fibrotic stage of empyema due to lack of one or more of the previous factors can still benefit from a trial of thoracoscopic decortication in specialized centres. VATS decortication has been shown to have superior outcomes for the treatment of persistent pleural collections in terms of postoperative morbidity, complications and length of hospital stay, and gives equivalent resolution when compared with open decortication (12,19).
One of the main cornerstones to success of using VATS in early stages of empyema is the education of the primary and secondary health care sectors; mainly chest physicians about the golden role of thoracoscopy for empyema. In a recent French questionnaire (20) to 87 medical physicians involved in treating complicated para-pneumonic effusions, only one (1/87) physician suggested surgery as the first stay of treating his patients. Good education aims to avoid misconceptions about the importance of surgery in early stage empyema and to avoid delayed referral which has a negative impact on outcomes of empyema treatment by VATS in both the pediatric (21) and adult population (9,22).
We believe all previous evidence should form the basis of performing a multi-centre randomized controlled trial (to avoid possible bias in our series) to compare surgical (VATS + antibiotics) and conservative management (chest drain + antibiotics) for stage I exudative empyema.
Until then or with more solid evidence regarding the use of intra-pleural fibrinolytics in early stage empyema, a routine thoracoscopic approach for all stages of empyema, particularly early stages, is justified as being the golden standard of management.
Conclusions
Thoracoscopic management of early stages of empyema should be the golden standard of management in surgically fit patients; particularly in the fibro-exudative phase of empyema. It is an effective and safe technique that reduces hospital stay, cost and avoids the need for a decortication via a thoracotomy in most cases.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: A new study application was permitted from the department of research in Ain Shams University (ASU-RG2-2013-17). An institutional research board approval (IRB) for the study was obtained.
References
- Rosenstengel A. Pleural infection-current diagnosis and management. J Thorac Dis 2012;4:186-93. [PubMed]
- Andrews NC, Parker EF, Shaw RR, et al. Management of nontuberculous empyema. Am Rev Respir Dis 1962;85:935-6.
- Wozniak CJ, Paull DE, Moezzi JE, et al. Choice of first intervention is related to outcomes in the management of empyema. Ann Thorac Surg 2009;87:1525-30; discussion 1530-1. [Crossref] [PubMed]
- Tong BC, Hanna J, Toloza EM, et al. Outcomes of video assisted thoracoscopic decortication. Ann Thorac Surg 2010;89:220-5. [Crossref] [PubMed]
- Chung JH, Lee SH, Kim KT, et al. Optimal timing of thoracoscopic drainage and decortication for empyema. Ann Thorac Surg 2014;97:224-9. [Crossref] [PubMed]
- Bagheri R, Tavassoli A, Haghi SZ, et al. The role of thoracoscopic debridement in the treatment of parapneumonic empyema. Asian Cardiovasc Thorac Ann 2013;21:443-6. [Crossref] [PubMed]
- Cassina PC, Hauser M, Hillejan L, et al. Video-assisted thoracoscopy in the treatment of pleural empyema: stage-based management and outcome. J Thorac Cardiovasc Surg 1999;117:234-8. [Crossref] [PubMed]
- Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc 2006;3:75-80. [Crossref] [PubMed]
- Lardinois D, Gock M, Pezzetta E, et al. Delayed referral and gram-negative organisms increase the conversion thoracotomy rate in patients undergoing video-assisted thoracoscopic surgery for empyema. Ann Thorac Surg 2005;79:1851-6. [Crossref] [PubMed]
- Kim SK, Kang CU, Song SH, et al. Factors predictive of the failure of medical treatment in patients with pleural infection. Korean J Intern Med 2014;29:603-12. [Crossref] [PubMed]
- Nakamura H, Taniguchi Y, Miwa K, et al. Surgical outcome of video-assisted thoracic surgery for acute thoracic empyema using pulsed lavage irrigation. Gen Thorac Cardiovasc Surg 2010;58:126-30. [Crossref] [PubMed]
- Bilgin M, Akcali Y, Oguzkaya F. Benefits of early aggressive management of empyema thoracis. ANZ J Surg 2006;76:120-2. [Crossref] [PubMed]
- Solaini L, Prusciano F, Bagioni P. Video-assisted thoracic surgery in the treatment of pleural empyema. Surg Endosc 2007;21:280-4. [Crossref] [PubMed]
- Chen KC, Chen HY, Lin JW, et al. Acute thoracic empyema: clinical characteristics and outcome analysis of video-assisted thoracoscopic surgery. J Formos Med Assoc 2014;113:210-8. [Crossref] [PubMed]
- Cameron R, Davies HR. Intra-pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Syst Rev 2008.CD002312. [PubMed]
- Rahman NM, Maskell NA, West A, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011;365:518-26. [Crossref] [PubMed]
- Colice GL, Idell S. Counterpoint: should fibrinolytics be routinely administered intrapleurally for management of a complicated parapneumonic effusion? Chest 2014;145:17-20. [Crossref] [PubMed]
- Scarci M, Zahid I, Billé A, et al. Is video-assisted thoracoscopic surgery the best treatment for paediatric pleural empyema? Interact Cardiovasc Thorac Surg 2011;13:70-6. [Crossref] [PubMed]
- Chambers A, Routledge T, Dunning J, et al. Is video-assisted thoracoscopic surgical decortication superior to open surgery in the management of adults with primary empyema? Interact Cardiovasc Thorac Surg 2010;11:171-7. [Crossref] [PubMed]
- Bénézit F, Letheulle J, Kerjouan M, et al. The management of complicated parapneumonic effusions in France. Rev Mal Respir 2015. [Epub ahead of print]. [PubMed]
- Velaiutham S, Pathmanathan S, Whitehead B, et al. Video-assisted thoracoscopic surgery of childhood empyema: early referral improves outcome. Pediatr Surg Int 2010;26:1031-5. [Crossref] [PubMed]
- Waller DA, Rengarajan A, Nicholson FH, et al. Delayed referral reduces the success of video-assisted thoracoscopic debridement for post-pneumonic empyema. Respir Med 2001;95:836-40. [Crossref] [PubMed]
Cite this article as: Elsayed HH, Mostafa A, Fathy E, Diab HS, Nofal IM, AbdelHamid OA, El-Bawab HY, ElNori AA. Thoracoscopic management of early stages of empyema: is this the golden standard? J Vis Surg 2018;4:114.


