Catastrophes and complicated intraoperative events during robotic lung resection
Introduction
Intraoperative complications and catastrophes are potential problems in any surgery. During anatomic pulmonary resection the close proximity of the tracheal-bronchial tree and pulmonary vasculature located in the small volume area in the center of the chest juxtaposed to the heart and great vessels create a unique set up for such events to potentially occur. Although the incidence or occurrence of such events, fortunately, is very uncommon during anatomic pulmonary resection be it via thoracotomy, VATS or robotics, these complications are responsible for nearly one quarter of the in-hospital mortalities (1-3). During the transition from thoracotomy to VATS, concerns were raised that the closed chest VATS approach was potentially problematic because to execute vascular control would require too much time to get into the chest. With the advent of robotic lobectomy, those same concerns and risks exist but now the surgeon is present only in the room but not scrubbed at the bedside.
While intraoperative complications/catastrophes are inevitable no matter how skilled the operative surgeon is, the experienced surgeon is always aware of their existence, tries to anticipate their development, is prepared to act in an instant but keen to prevent such problems from occurring (3). And, while all thoracic surgeons are prepared to tell you about “the time such and such happened”, the literature documenting the incidence and articulating the solutions to these events is sparse regardless of surgical approach. These types of complications are not captured by any major society or administrative database (2) and the individual surgeon or team is unlikely to have a large series. As such research attempting to delineate the incidence and causative factors is nearly impossible to perform.
In this paper, the focus will be on intraoperative pulmonary artery and vein injuries, major airway injuries, inadvertent transections, injuries to major abdominal organs and effects of carbon dioxide insufflation during robotic pulmonary resection. Given the similarity with VATS lobectomy and the sparse literature, experiences and solutions for similar events are presented from both approaches in order to draw upon the entire minimally invasive experience.
Pulmonary vascular injury
Pulmonary arterial injury
The most common intraoperative catastrophe during anatomic pulmonary resection is an injury to the pulmonary artery (Table 1). The incidence is reported to occur in 0.5% to 2.6% in series of greater than 100 robotic lobectomies (1,4-8) and from 1 to 2.9% of VATS lobectomy (2,3,9,10). In most cases, this injury was also the primary reason for emergent/urgent conversion from a minimally invasive approach to thoracotomy. In most series the minority of cases were being managed with a minimally invasive approach (1,3). However, one VATS series suggests that over 80% can be managed minimally invasively with a novel technique of angiorrhaphy (see below) (10). The upper lobes were the most common site of injury during robotic cases owing to the multiple arterial branches on the left, the large truncus on the right and the fact that these sites are favored in lung cancer (1,3).
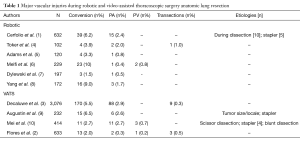
Full table
Injuries to the pulmonary arterial system occur from a variety of events and situations. Surgeon experience does not seem to influence the incidence of this injury (3). Most commonly it appears to occur during blunt and sharp “dissection” of the artery but it is not always reported under which circumstances this might be occurring. It is recognized that patients receiving induction chemo and/or radiation therapy and larger tumor size are at greater risk for an arterial injury though the numbers are small (3,9). Injuries are also noted to occur around the time an endovascular stapler is applied and fired leading to staple line bleeding or more central tears (1,9,10). Lastly, the presence of calcified lymph nodes requiring dissection also creates risk for an injury (2).
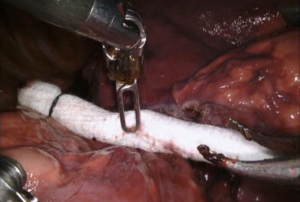
When an injury occurs, the initial response from most surgeons is one of fright and a surge of catecholamine. In series that described their management of an injury, all cited the need to remain calm, poised and in charge (1,10,11). The first step is applying pressure on the injury. This can be accomplished with the overlying lung, using one or two pre-rolled sponges (1), inserting a sponge stick or using pressure via a blunt tipped suction device (10) (Figure 1). After obtaining control, its crucial to inform the anesthesiologist, nursing team and request assistance from surgical partners is critical. We have found as have others (1) that maintaining pressure for 5–7 minutes by the clock often allows the team to get organized but it also allows one to differentiate the degree of injury since some will stop with simple pressure.
When the team is ready, pressure control can be transferred to an external bedside assistant and the camera port undocked so the camera is free to maintain a visual on the site. Then, the remaining robotic instruments can be removed, the robot undocked and safely moved aside. A standard posterolateral thoracotomy can be performed under controlled circumstances while pressure is applied and the camera to visualize is left in place. Once inside the thoracic cavity, surgeons can proceed as they would in an open situation—proximal and distal control followed by a determination on repair, ligation or transection.
In the majority of cases and surgeons, there is no other option except to proceed with conversion to thoracotomy in hopes of salvaging the resection, preserving life and then lung parenchyma. With increasing experience, we have observed, as have others, that the sponge and pressure can be slowly released to see what volume of bleeding ensues (Figure 2). If the bleeding has stopped the surgery could simply continue. Or, if minimal or persistent low volume bleeding continues and the injury can be discerned it may be possible to control the injury with surgical clips, stapling more proximally or intra-corporeal suturing. This decision requires weighing multiple factors such as the patient’s status, oncologic outcomes, access and feasibility and the threat to patient life.

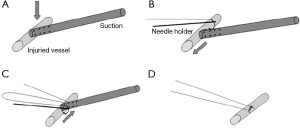
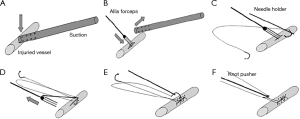
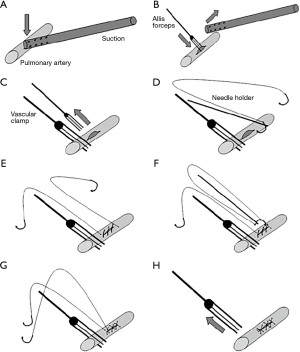
Mei and colleagues (10,13) recently reported on a novel sequential VATS technique that allowed over 80% of vascular injuries to be controlled minimally invasively. This requires control with pressure from a suction device. This can then be followed by placement of a series of sutures on either side of the suction device allowing the injury to be closed (Figure 3). Alternatively, the suction device is replaced with an Allis clamp for control followed by mattress sutures (Figure 4). In extreme circumstances, a vascular clamp is applied proximally with the Allis clamp for greater control followed by sutures. (Figure 5). This technique may be translatable to robotic lobectomy but require an additional port to allow the Allis clamp to be inserted and the surgeon will require skills to suture inside the chest.
Pulmonary vein injury
Injury to a pulmonary vein is much less common than a pulmonary arterial injury. Several series have reported its occurrence but no details are provided on the etiology (2,6,10). In one VATS case, a staple line dehiscence occurred when the pericardium was inadvertently caught in the staples. This was managed with a thoracotomy and intrapericardial oversewing of the defect (2).
Great vessel and thoracic duct injury
The superior vena cava, azygous vein, thoracic aorta and thoracic duct are all structures that reside within the thoracic cavity in continuity with the lung. Therefore, they are potential structures that may be injured during robotic lung resection. Fortunately, since the majority of minimally invasive lung resections are carried out for relatively early stage disease, these structures are rarely injured. However, with increasing experience and as surgeon’s tackle more advanced disease, these structures will be potential structures that can be injured.
The most commonly injured structure is the thoracic duct. This usually occurs as a result of an extensive lymphadenectomy in the subcarinal region as the duct passes from its position between the aorta, the azygous vein and the vertebral bodies in the right chest to cross and ascend toward the left subclavian vein. Occasionally, it will be exposed during decortication from a prior pleural process during mobilization of the lung. If chyle is identified during resection, the duct can be directly clipped or ligated or alternatively, en mass ligature at the aortic hiatus can be performed. More commonly, it is identified as a modestly high output chest tube drainage that turns milky with institution of oral diet. Standard treatment algorithms apply but we tend to favor early return to the operating room for ligation when the output approaches or is greater than 500 mL/day.
Although injuries have been reported to the azygous vein and superior vena cava during minimally invasive lung resection the true incidence is unknown (2,10). In one reported series, an injury to the azygous-caval junction occurred during resection of station 4R lymph nodes and was repaired successfully by thoracotomy. The mechanism was not reported but it is possible that this was related to traction or thermal/cautery injury (10). In the other series, two superior vena caval injuries are reported during right lower lobectomy for which both were repaired via a VATS approach. Unfortunately, no further details were provided.
Erroneous transections
Inadvertent transections or divisions of uninvolved structures in the pulmonary hilum occur primarily in situations with distorted anatomy due to scarring or a centrally placed tumor. In three VATS transections involving the proximal or main pulmonary artery, the incident was recognized immediately. In each case the patient underwent thoracotomy with resection of the tumor. In two cases, the arterial supply was reconstructed and in the remaining case a pneumonectomy was required (2). In one robotic series, an inadvertent transection of the pulmonary artery occurred during a resection after chemoradiotherapy to 60 Gy. This patient underwent thoracotomy and sleeve resection of the pulmonary artery (4).
The pulmonary vein is also prone to inadvertent transection. In one VATS series the middle lobe vein was most commonly the structure transected for no apparent reason other than failure of recognition; however, when an upper or lower vein was transected the common finding was either a centrally placed tumor and/or the use of induction chemoradiotherapy (3). Most authors noted the importance of clearly identifying and delineating the lower lobe vein as a separate entity from the upper vein as one method for avoiding an erroneous transection. Once the injury occurred, a thoracotomy was performed and the lower or upper veins were reimplanted if appropriate. If the middle lobe vein was transected, conversion was not performed but bilobectomy was completed (3).
Inadvertent transections of the airway have also been reported. Mostly commonly the bronchus intermedius was transected during lower lobectomy necessitating bilobectomy (3). In another VATS series, the middle lobe bronchus was divided during upper lobectomy due to a challenging anterior fissure. This also necessitated a bilobectomy (2).
Tracheal-bronchial airway injury
An injury to the uninvolved airway, proximal trachea or contralateral main stem bronchus is unusual and rare. In the reported series, the most common etiology was the double lumen endotracheal tube causing a tear in the main bronchus either from over inflation of the balloon and during manipulation of the tube. However, injuries have also been reported to occur during dissection around the middle lobe during VATS bilobectomy, during stapling of the lower lobe bronchus and nodal dissection in the subcarinal space along the bronchus intermedius (2,3). These were all managed by thoracotomy, primary repair with buttress or more proximal resection.
Gastrointestinal organ injury
Injuries to the adjacent esophagus or sub diaphragmatic liver and spleen are uncommon. The esophagus can become involved as in innocent bystander during nodal dissection in the subcarinal (station 7) or station 9 usually from an electrocautery injury and less commonly from direct laceration (3). In one reported case, VATS nodal dissection was thought to be the causal factor leading to an esophago-bronchial fistula 6 weeks after resection. This was initially treated with a thoracotomy and muscle interposition (2). Occasionally, the stapler tip has been reported to be the cause of inadvertent trauma to the esophagus (3). Treatment depends on the severity of injury and can involve simple suture closure to formal two-layer repair with a buttress reinforcement flap.
Solid organ injuries primarily to the spleen but also the liver occur rarely. These injuries are thought to be caused by low port placement, misaligned stapler tips entering the chest and cautery arcing via the diaphragm (2). We favor placing the most anterior port (6–7th intercostal space, anterior axillary line), which becomes our chest tube site, as the first port so that lower ports are placed under direct vision and hopefully avoids these rare injuries. Treatment options depend on the injury and blood loss but include observation, embolization and lastly operative splenectomy/splenorrhaphy and packing (3).
Miscellaneous complications
There are a variety of very unusual or rare complications that necessitate further surgery that most thoracic surgeons are aware of but are rarely reported. These include lobar torsion, massive parenchymal air leak after decortication in preparation for resection and airway kinking (3). The treatment of these complications is not standard and based on individual surgeon judgment. Lastly, cardiac arrhythmias occasionally occur such as ventricular tachycardia or atrial fibrillation (3).
Effects of CO2 insufflation
One unique feature of robotic lobectomy is that CO2 insufflation is often used particularly during completely portal procedures. During VATS resection this is rarely used. As such several complications can arise from its use including CO2 embolus, compromised venous return, severe brachycardia or progressive arterial desaturation and acid-base disturbances secondary to hypercarbia (14). It is important that thoracic surgeons performing robotic surgery with CO2 insufflation be aware of these rare events because as the laparoscopic surgeons have discovered these occur rapidly as in the case of CO2 embolus or insidiously over the course of the case creating physiologic disturbances that can prevent extubation. These events can be limited by keeping the flow and set pressure of CO2 as low as possible to allow for visualization. Often, once the lung is deflated the need for CO2 is negligible and can be turned off. One additional reason to stop the flow of CO2 early is that in a swine model it appears to limit blood loss via application of pressure on the vessels which when released can potentially bleed (15).
Conclusions
Intraoperative complications and catastrophes during pulmonary resection are uncommon but can result in significant consequences for the patient. There is a paucity of reported experiences during robotic lobectomy. Even in the more mature VATS lobectomy experience, these complications are very uncommon. Robotic surgeons regardless of experience should have a “fire drill” plan for the rare event so that the team members understand their roles during these events. To increase learning and understanding VATS and robotic lung surgeons are encouraged to pool their results and report these events and their management.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Louie reports a prior and current relationship with Intuitive Surgical and is currently a recipient of a restricted research grant from Intuitive Surgical.
References
- Cerfolio RJ, Bess KM, Wei B, et al. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann Thorac Surg 2016;102:394-9. [Crossref] [PubMed]
- Flores RM, Ihekweazu U, Dycoco J, et al. Video-assisted thoracoscopic surgery (VATS) lobectomy: catastrophic intraoperative complications. J Thorac Cardiovasc Surg 2011;142:1412-7. [Crossref] [PubMed]
- Decaluwe H, Petersen RH, Hansen H, et al. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588-98; discussion 599. [Crossref] [PubMed]
- Toker A, Özyurtkan MO, Kaba E, et al. Robotic anatomic lung resections: the initial experience and description of learning in 102 cases. Surg Endosc 2016;30:676-83. [Crossref] [PubMed]
- Adams RD, Bolton WD, Stephenson JE, et al. Initial multicenter community robotic lobectomy experience: comparisons to a national database. Ann Thorac Surg 2014;97:1893-8; discussion 1899-900.
- Melfi FM, Fanucchi O, Davini F, et al. Robotic lobectomy for lung cancer: evolution in technique and technology. Eur J Cardiothorac Surg 2014;46:626-30; discussion 630-1. [Crossref] [PubMed]
- Dylewski MR, Ohaeto AC, Pereira JF. Pulmonary resection using a total endoscopic robotic video-assisted approach. Semin Thorac Cardiovasc Surg 2011;23:36-42. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-437. [Crossref] [PubMed]
- Augustin F, Maier HT, Weissenbacher A, et al. Causes, predictors and consequences of conversion from VATS to open lung lobectomy. Surg Endosc 2016;30:2415-21. [Crossref] [PubMed]
- Mei J, Pu Q, Liao H, et al. A novel method for troubleshooting vascular injury during anatomic thoracoscopic pulmonary resection without conversion to thoracotomy. Surg Endosc 2013;27:530-7. [Crossref] [PubMed]
- Berry MF. Pulmonary Artery Bleeding During Video-Assisted Thoracoscopic Surgery: Intraoperative Bleeding and Control. Thorac Surg Clin 2015;25:239-47. [Crossref] [PubMed]
- Louie BE. Pressure with rolled sponges can facilitate control of bleeding and allow for stapler division. Courtesy of Dr. Robert Cerfolio. Asvide 2017;4:141. Available online: http://www.asvide.com/articles/1449
- Xiao ZL, Mei JD, Pu Q, et al. Technical strategy for dealing with bleeding during thoracoscopic lung surgery. Ann Cardiothorac Surg 2014;3:213-5. [PubMed]
- Campos J, Ueda K. Update on anesthetic complications of robotic thoracic surgery. Minerva Anestesiol 2014;80:83-8. [PubMed]
- Okamura R, Takahashi Y, Dejima H, et al. Efficacy and hemodynamic response of pleural carbon dioxide insufflation during thoracoscopic surgery in a swine vessel injury model. Surg Today 2016;46:1464-70. [Crossref] [PubMed]
Cite this article as: Louie BE. Catastrophes and complicated intraoperative events during robotic lung resection. J Vis Surg 2017;3:52.