Primary squamous cell carcinoma of the peristomal skin of gastrostomy in a transplant patient: a first case report
Introduction
Gastrostomy tube placement is a commonly method to provide the enteral nutrition when oral eating is not possible or not sufficient. Gastrostomy tube is usually placed endoscopically [percutaneous endoscopic gastrostomy (PEG)]. The reported rates of complications varied from 16% to 70% (1,2). The majority of these complications are minors, included peristomal wound infection, stomal leakage, buried bumper, gastric ulcer, ileus, fistulous tracts and inadvertent removal (3). In rare cases, tumor implantation at gastrostomy site has been reported, especially in patients with head and neck cancer (4). Furthermore, primary squamous cell carcinoma (SCC) of the peristomal skin of a gastrostomy tube was previously reported in two cases in the literature (5,6). We described in this report the first case of SCC in peristomal skin of a gastrostomy in a transplanted patient.
Case presentation
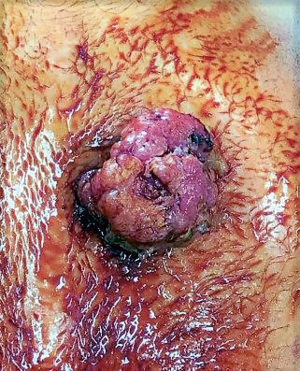
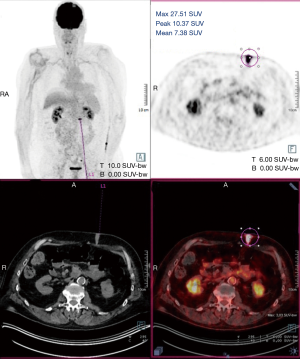
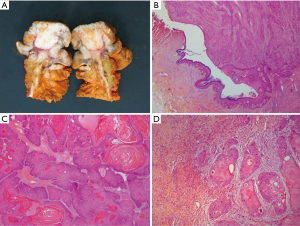
A 60-year-old man who underwent a combined heart-liver transplantation in 2013 for hereditary amyloidosis, developed several infectious complications after transplantation leading to severe malnutrition. In 2016, due to his nutritional status, a PEG tube was placed for enteral feeding. After 18 months, the gastrostomy tube was removed because the patient’s nutritional status has improved. Few months later, he presented in our department for persistent gastrostomy site leakage with local skin infections. Despite a local care therapy, the gastrocutaneous fistula persisted which impacted on quality of life (Figure 1). Because the conservative approach failed, we planned to perform a surgical excision of the gastrostomy site. The immunosuppressive regimen was changed by switching everolimus for tacrolimus to avoid surgical complications related to tissue healing. Two months after stopping Everolimus, the granulation considerably increased associated with a poor general condition. Computed tomography revealed a tumoral process at the expense of the gastrostomy site associated with a gastrocutaneous fistula. Gastric endoscopy was performed and not found a gastric invasion. Positron emission tomography scan revealed an isolated high metabolism of the tumor (standard uptake value =10.4) with no other anomaly (Figure 2). We performed a surgical resection of the tumor and surrounding abdominal wall and submitted for histological analysis. No sign of local extension to adjacent organs was found during the procedure. The postoperative course was uneventful and the patient was discharged in postoperative day 7. The pathological report of the specimen revealed a 5.5-centimeter well-differentiated squamous cell skin carcinoma (SCC) with tumor-free margins (Figure 3). Everolimus treatment was resumed after surgery with close follow-up.
Discussion
SCC is a common form of skin cancer that develops in the squamous cells. It’s also considered as the most common cutaneous malignancies (7) and the major cause of morbidity after organ transplantation (8). Furthermore, the incidence of SCC is significantly increased in organ transplant recipients, especially in patients with heart or lung transplants (9). The main risk factors of SCC include sun chronic exposure, viral infections (human papillomaviruses), male sex, older age and smoking (10). Chronic cutaneous inflammation is also recognized as one important player in the development of SCC (11). Thus, the peristomal skin of gastrostomy is usually characterized by a local skin irritation due to gastric secretions leading to chronic inflammation.
Two similar cases have been reported, describing a primary SCC of peristomal skin of gastrostomy. Oh et al. reported the case of a 24-year-old man with a seizure disorder requiring enteral nutrition by gastrostomy since his second year of life. He developed a large amount of granulation tissue around the gastrostomy site, treated for 9 months with nitrate and local care. Excision of this granulation revealed a SCC (5). The second case concerned a 73-year-old man who suffered esophageal chemical burns after swallowing sodium hypochlorite 50 years earlier that was initially managed with esophageal exclusion and placement of a gastrostomy for enteral feeding. He presented with an exophytic and painful mass of the skin adjacent to his gastrostomy site. Biopsy of the mass revealed a SCC (6). These two cases had similarities and concerned patients with a prolonged gastrostomy use (respectively 22 and 50 years). Unlike these cases, our patient developed a SCC after only 2 years of enteral nutrition by gastrostomy tube. Moreover, in the current case, the granulation significantly increased 2 months after stopping Everolimus treatment. To our knowledge, this is the first report of a primary SCC arising from a gastrostomy tube site in a transplant patient.
After organ transplantation, immunosuppressive therapy is necessary to prevent rejection but also increases the risk of skin cancer formation. For example, cyclosporine promotes the tumor growth via VEGF-mediated angiogenesis and inhibits DNA repair after ultraviolet-induced skin damage. Furthermore, other immunosuppressive medication, such as azathioprine, sensitizes DNA to ultraviolet-A radiation and increase the risk of malignant transformation (7).
It was noteworthy that tumor progression was correlated with the use of everolimus. It is one of the mammalian target of rapamycin (mTOR) inhibitors with an antitumoral effect (12). The mTOR pathway is a major mechanism of cellular growth and homeostasis and is associated with risk reduction in de novo malignancy. mTOR inhibitors have also been associated with a decreased risk of developing non-melanoma skin cancers in comparison to calcineurin inhibitors (7,13). In our case, we hypothesize that development of SCC was attributed to a chronic inflammation in the skin surrounding the gastrostomy site in an immunocompromised patient after interruption of Everolimus treatment.
In conclusion, the development of carcinoma in a gastrostomy site is rare but may be increased in organ transplant recipients. This is attributed to the immunosuppressive drugs and significantly increase morbidity and mortality. We must take special attention to the risk of SCC in these immunocompromised patients by regular monitoring the entire skin coating and especially the various sites of prolonged cutaneous trauma.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Blomberg J, Lagergren J, Martin L, et al. Complications after percutaneous endoscopic gastrostomy in a prospective study. Scand J Gastroenterol 2012;47:737-42. [Crossref] [PubMed]
- Keung EZ, Liu X, Nuzhad A, et al. In-hospital and long-term outcomes after percutaneous endoscopic gastrostomy in patients with malignancy. J Am Coll Surg 2012;215:777-86. [Crossref] [PubMed]
- Lynch CR, Fang JC. Prevention and management of complications of percutaneous endoscopic gastrostomy (PEG) tubes. Pract Gastroenterol 2004;28:66-76.
- Fung E, Strosberg DS, Jones EL, et al. Incidence of abdominal wall metastases following percutaneous endoscopic gastrostomy placement in patients with head and neck cancer. Surg Endosc 2017;31:3623-7. [Crossref] [PubMed]
- Oh PS, Gill KZ, Lynch LJ, et al. Primary squamous cell carcinoma arising at a gastrostomy tube site. J Pediatr Surg 2011;46:756-8. [Crossref] [PubMed]
- Cerdán Santacruz C, Díaz Del Arco C, Rubio Herrera MÁ, et al. Squamous Cell Carcinoma of the Peristomal Skin of a Gastrostomy: Case Study. J Wound Ostomy Continence Nurs 2017;44:384-6. [Crossref] [PubMed]
- Chockalingam R, Downing C, Tyring SK. Cutaneous Squamous Cell Carcinomas in Organ Transplant Recipients. J Clin Med 2015;4:1229-39. [Crossref] [PubMed]
- Adami J, Gäbel H, Lindelöf B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer 2003;89:1221-7. [Crossref] [PubMed]
- Krynitz B, Edgren G, Lindelöf B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008--a Swedish population-based study. Int J Cancer 2013;132:1429-38. [Crossref] [PubMed]
- Dreno B. Skin cancers after transplantation. Nephrol Dial Transplant 2003;18:1052-8. [Crossref] [PubMed]
- Gomes M, Teixeira AL, Coelho A, et al. The role of inflammation in lung cancer. Adv Exp Med Biol 2014;816:1-23. [Crossref] [PubMed]
- Dumortier J, Dharancy S, Calmus Y, et al. Use of everolimus in liver transplantation: The French experience. Transplant Rev (Orlando) 2016;30:161-70. [Crossref] [PubMed]
- Jung JW, Overgaard NH, Burke MT, et al. Does the nature of residual immune function explain the differential risk of non-melanoma skin cancer development in immunosuppressed organ transplant recipients? Int J Cancer 2016;138:281-92. [Crossref] [PubMed]