Clinical fate of T0N1 esophageal cancer: results from the National Cancer Database
Introduction
Esophageal cancer (EC) is the eighth most common cancer worldwide and one of the fastest growing cancer diagnoses in the United States with 16,940 new cases and 15,690 deaths estimated for 2017 (1,2). Advancements in the treatment of EC has improved median survival from 11 months in the 1970s to 35 months after the year 2000 (3). Even with developments in surgical technique and neoadjuvant therapy, EC patients have a 10-year survival rate of only 14% (3).
Surgical resection has become a mainstay of EC therapy with a goal of achieving negative surgical margins (4). The addition of neoadjuvant chemoradiation (NCR) has demonstrated a decrease in pathological node positive status, decreased positive surgical margins, and lesser rate of recurrence (5,6). Most notably, neoadjuvant therapy has improved median survival to 49.4 versus 24.0 months for surgery alone (7). Furthermore, neoadjuvant therapy has improved pathologic complete response (pCR) rates (8). Throughout the history of EC treatment pCR has been established to have the most improved median and overall survival (OS) up to 66 months and 52% respectively (8). Achieving pCR has also demonstrated improved R0 resection rates and decreased rates of recurrence when compared to pathologic partial response (pPR) and non-responders (NR) (8).
In addition to neoadjuvant therapy, adjuvant therapy for EC continues to be investigated but controversial (4,9,10). One study did demonstrate a survival benefit in node positive patients who received adjuvant therapy and using multivariate analysis they demonstrated that adjuvant therapy is an independent prognostic factor in survival for node positive patients (9).
Pathological complete response rate is well understood in terms of survival and positive-node status has shown improvement with adjuvant therapy. However, patients with pathological complete response of the primary tumor (T0) but positive nodal status (N1) have an undetermined outcome. Our goal is to determine the significance of a T0N1 diagnosis, the overall prognosis for these patients and whether they will benefit from additional therapy.
Methods
The National Cancer Database (NCDB) is a dataset maintained by the American College of Surgeons and the American Cancer Society and collects patient data from >1,500 centers across the United States. This retrospective database study was approved and deemed exempt by the institutional review board at Sarasota Memorial Hospital as it did not involve patient identifiers. Utilizing the National Cancer Database, we identified patients with EC between 2004 and 2013 who underwent neoadjuvant chemotherapy and radiation to a total dose between 41.4–50.4 Gy followed by esophagectomy. We further identified those patients who had subsequent pathology of T0N1. Patients were excluded if they had metastatic disease, cervical EC, unknown nodal status, or if mortality occurred within 90 days. Only adenocarcinoma (AC) and squamous cell carcinomas (SCC) were included.
Patient characteristics were reported by group using mean, median, SD and interquartile range for continuous variables and using frequencies for categorical variables. Baseline univariate comparisons of patient characteristics were made for continuous variables using both the Mann-Whitney U and Kruskal Wallis tests as appropriate. Pearson’s Chi-square test was used to compare categorical variables. Survival was evaluated on the basis of time from date of diagnosis to date of death or censoring. Unadjusted survival analyses were performed using the Kaplan-Meier method comparing survival curves with the log-rank test. All statistical tests were two-sided and α (type I) error <0.05 was considered statistically significant. Statistical analysis was performed using SPSS® version 23.0 (IBM®, Chicago, IL, USA). This study was approved as exempt by the Institutional Review Board.
Results
We identified 7,116 patients with EC; 6,235 (87.6%) with AC and 881 (12.4%) with SCC. The average age of both groups was 62 years and there were more males in both groups, 5,453 (87.5%) men with AC and 578 (65.6%) men with SCC. The median lymph nodes removed were 13 [0–83] and the mean lymph nodes positive were 1.4±2.9. Negative surgical margins were achieved in 6,668 (93.7%) of patients and positive margins in 448 (6.3%). Complete response rates were identified in 1,334 (18.7%), partial response in 2,812 (39.5%), and no response in 2,970 (41.7%). Of all patients, 763 (10.7%) received adjuvant chemotherapy. Adjuvant therapy was received in 699 (11.2%) of AC group and 64 (7.3%) of SCC. A total of 230 patients (3.2%) were diagnosed as T0N1 (Table 1).
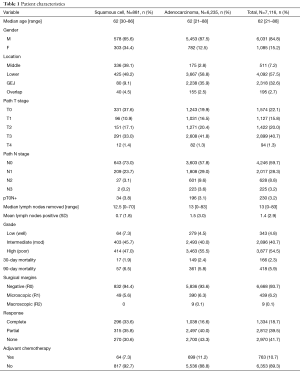
Full table
OS was greatest in patients with pathological complete response; median OS 61.7 months and 5-year OS 51.8%. Node negative patients (N0) also showed improved median and OS; 54.2 months and 46.9% respectively. Patients with R0 surgical margins showed a 39.5 month median survival and 38.6% 5-year survival and R1/2 resected patients had the worst median and OS of 20.1 months and 14.1% respectively, P<0.001.
Patients with T0N1 demonstrated a median OS of 32.1 months and 5-year OS of 26.4% P<0.001 compared to patients with pCR (61.7 months and 51.8%). Patients with T1/2N0 had better median and OS compared to T1/2N+; 58.2 months and 48.5% versus 30.5 months and 24.9% respectively, P<0.001 (Table 2).
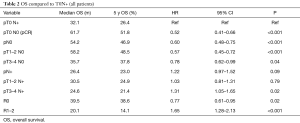
Full table
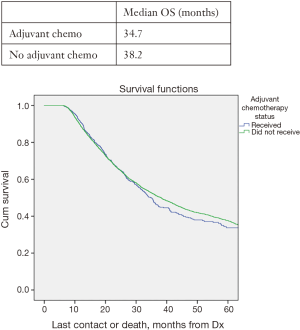
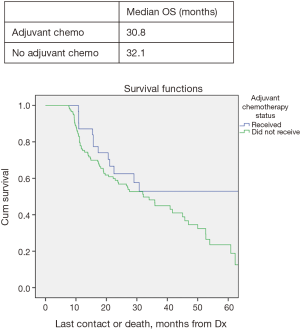
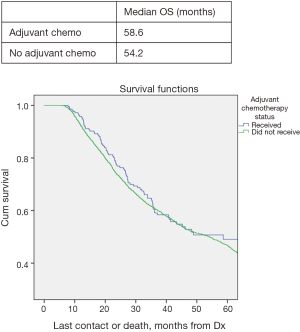
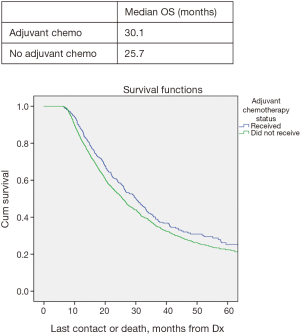
In all patients, there was no significant improved median OS with adjuvant therapy versus no adjuvant therapy, P=0.4 (Figure 1). Patients with T0N1 who received adjuvant therapy also failed to reach statistically significant improved OS compared to no adjuvant therapy, P=0.08 (Figure 2). Node negative patients similarly did not show improved median and OS with adjuvant therapy, P=0.4 (Figure 3). However, node positive patients did show greater median and OS with adjuvant therapy 30.1 months and 27% than without adjuvant therapy 25.7 months and 24.3%, P=0.03 (Figure 4). Multivariate analysis demonstrated that in patients with T0N1 the number of lymph nodes positive and histology were independent predictors of survival (P<0.001) (Table 3).
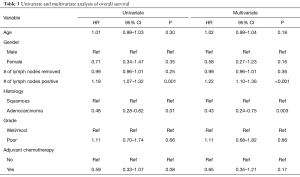
Full table
Discussion
Our study demonstrates that a pathologic T0N1 diagnosis has a 5-year survival of 26.4% which is worse than the prognosis of pCR rate. We identified that node-positive patients have statistically improved survival with adjuvant therapy versus their non-adjuvant therapy counterparts. However, patients with pathologic T0N1 diagnosis had no significant improved survival with the addition of adjuvant therapy.
The current National Comprehensive Cancer Network (NCCN) guidelines for EC treatment recommend neoadjuvant therapy prior to surgical excision (11). In 2006, the MAGIC trials published an investigation of 503 patients who either received neoadjuvant chemotherapy or surgery alone for EC. The MAGIC trials clearly demonstrated improved 5-year survival with neoadjuvant chemotherapy versus surgery alone, 36% and 23% respectively (12). NCR was further studied in the CROSS trials which demonstrated improved median survival in patients receiving NCR versus surgery alone, 49.4 and 24.0 months respectively (6,7).
Neoadjuvant therapy has the potential to produce pathological complete response in EC. According to investigations, pathological complete response rates can range between 26% and 40.5% with an associated 5-year survival of up to 55% (8,13,14). Our results showed slightly lower pathological complete response rate but patients who achieved pCR did have significantly improved median and OS. With the discovery of pathological complete responses from neoadjuvant therapy, there has been an increased interest in patients who achieve complete response of the primary tumor but retain positive nodal status. Few studies have investigated the T0N1 patients and produced varied results, likely due to very small sample size (15,16). Our larger study demonstrated T0N1 prognosis being more similar to T1/2N+ prognosis in terms of survival.
Adjuvant therapy is currently under scrutiny as an addition to neoadjuvant therapy and esophagectomy for EC. In 2003, Xiao et al. did a prospective randomized trial of 495 patients with EC, 275 who received surgery alone and 220 who received surgery with adjuvant chemotherapy. The 5-year survival between the two groups did not reach statistical significance. Interestingly, there was statistically improved 5-year survival in patients with node-positive status who received adjuvant therapy versus those who did not, 29.2% and 14.7% respectively (17).
A recent study by Brescia et al. retrospectively investigated 764 EC patients who received induction therapy and esophagectomy with positive nodal status on pathology. Forty-five of the patients received adjuvant therapy and they discovered improved median OS of patients who received adjuvant therapy versus those who did not, 24 versus 18 months respectively (18). It has become more evident that node-positive patients benefit from adjuvant therapy and our study similarly found improved survival in node-positive patients who received adjuvant therapy. We sought to identify whether those with positive nodes but complete response of the primary tumor would also benefit from adjuvant therapy. However, our study was unable to reach statistical significant improved survival for patients who achieved pathological complete response rates but with positive lymph nodes.
Conclusions
The addition of adjuvant therapy is beneficial for node positive patients but shows no benefit for patients with T0N1 upon post-operative pathology. Unfortunately, the diagnosis of T0N1 has worse survival outcomes than a diagnosis of pathological complete response and has no better survival than T1/2 patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Dubecz A, Gall I, Solymosi N, et al. Temporal trends in long-term survival and cure rates in esophageal cancer: a SEER database analysis. J Thorac Oncol 2012;7:443-7. [Crossref] [PubMed]
- Purwar P, Bambarkar S, Jiwnani S, et al. Multimodality management of esophageal cancer. Indian J Surg 2014;76:494-503. [Crossref] [PubMed]
- Markar SR, Arya S, Karthikesalingam A, et al. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann Surg Oncol 2013;20:4274-81. [Crossref] [PubMed]
- Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385-91. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Meredith KL, Weber JM, Turaga KK, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol 2010;17:1159-67. [Crossref] [PubMed]
- Shridhar R, Almhanna K, Meredith KL, et al. Radiation therapy and esophageal cancer. Cancer Control 2013;20:97-110. [Crossref] [PubMed]
- Tachibana M, Yoshimura H, Kinugasa S, et al. Postoperative chemotherapy vs. chemoradiotherapy for thoracic esophageal cancer: a prospective randomized clinical trial. Eur J Surg Oncol 2003;29:580-7. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers. 2013.
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [see comment]. [Crossref] [PubMed]
- Donahue JM, Nichols FC, Li Z, et al. Complete pathologic response after neoadjuvant chemoradiotherapy for esophageal cancer is associated with enhanced survival. Ann Thorac Surg 2009;87:392-8; discussion 398-9. [Crossref] [PubMed]
- Rizvi FH, Syed AA, Khattak S, et al. Complete pathological response after neoadjuvant treatment in locally advanced esophageal cancer predicts long term survival: a retrospective cohort study. Int J Surg 2014;12:621-5. [Crossref] [PubMed]
- Kim MP, Correa AM, Lee J, et al. Pathologic T0N1 esophageal cancer after neoadjuvant therapy and surgery: an orphan status. Ann Thorac Surg 2010;90:884-90; discussion 890-1. [Crossref] [PubMed]
- Blackham AU, Yue B, Almhanna K, et al. The prognostic value of residual nodal disease following neoadjuvant chemoradiation for esophageal cancer in patients with complete primary tumor response. J Surg Oncol 2015;112:597-602. [Crossref] [PubMed]
- Xiao ZF, Yang ZY, Liang J, et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg 2003;75:331-6. [Crossref] [PubMed]
- Brescia AA, Broderick SR, Crabtree TD, et al. Adjuvant Therapy for Positive Nodes After Induction Therapy and Resection of Esophageal Cancer. Ann Thorac Surg 2016;101:200-8; discussion 208-10. [Crossref] [PubMed]