
Correlation between vascular endothelial growth factor-A expression and tumor location and invasion in patients with colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most prevalent and the fourth leading cause of cancer death in the world. CRC represented the fifth commonest cancer in Indonesia (1). The degree of tumor vascularization reflects the evolution of CRC. As in the majority of solid tumors, angiogenesis is essential for tumor development and for its loco-regional and metastatic growth (2,3). When neoplastic mass is supported only by the host vessels, it stays in a limited size with slow propagation. In contrary, when the formation of new blood vessels is supported by the neoplastic cells through the secretion of proangiogenic factors, nutrients from the newly formed capillaries stimulate a more rapid growth and progression of the tumor (4,5). Degree of tumor angiogenic activity was considered to determine tumor aggressiveness (6). Vascular endothelial growth factor-A (VEGF-A) is the most potent mediator in the process of angiogenesis, contributing to migration, proliferation, and tube formation of endothelial cells (7). Tumor neovascularization can further be assessed by the measurement of microvessel density (MVD). The MVD has long been observed as a surrogate marker which expressly reflects tumor angiogenesis in various cancers, including CRC (8,9).
Association between angiogenic markers and clinical characteristics and outcome remains controversial. Several studies showed that VEGF positively correlated with sex, age, clinical stage, tumor histology grade, tumor depth (T), nodal metastasis, and distant metastasis (10-13), although others did not confirm the findings (14-16). Many retrospective studies have demonstrated that VEGF and MVD can be used as biological markers to predict survival in patients with CRC. High level of VEGF expression has a relationship with poorer overall- and disease free-survival (8,10,17-20), as well as an increased rate of distant metastases (21). High MVD predicts an unfavorable overall survival (OS) and disease-free survival (8,22-24). Some studies also demonstrated an association between an increase expression of VEGF with higher MVD (25-27), although others paradoxically found no association (22,28).
Only few data on angiogenesis according to clinicopathological features and survival outcome have been reported in Indonesian patients with CRC (12). This study aimed to examine pathological expression of VEGF-A and MVD measurement and to investigate a correlation between both markers with clinicopathological features and to analyze their prognostic influence on patients’ survival. Data from the local hospital will yield a clearer view of angiogenesis patterns in samples of Indonesian patients. This approach also may provide more information to answer the lack of certainty of contradictive results obtained from previous reports.
Methods
Patients
This retrospective study included patients with CRC who were treated between 2007 and 2013 at Dr. Sardjito General Hospital Yogyakarta. Patients were histopathologically diagnosed with adenocarcinoma type following surgical resection. Demographical, clinical, and pathological data were reviewed from the patients’ medical records. Subjects were excluded when only minimal information was available or when the corresponding biopsy samples did not meet the quality criteria for staining. All patients had not undergone any treatment prior to the resection of the primary tumour. The study was approved by the Ethics Committee of Faculty of Medicine, Public Health, and Nursing Universitas Gadjah Mada/Dr Sardjito Hospital Yogyakarta (reference: KE/FK/209/EC). Informed consent was obtained from study subjects.
Data from patients’ records were collected to assess clinical and histopathological variables. Data were then categorized according to age (≤ median vs. > median), sex (male vs. female), tumor location (colon vs. rectum), histology differentiation grade (well differentiation vs. moderate to poor differentiation), T invasion (T1–3 vs. T4), nodal metastasis (N0 vs. N1–4), and the presence of metastasis at baseline (M0 vs. M1).
Immunohistochemistry
The cancer tissue was obtained as formalin-fixed paraffin-embedded samples from the archives of the Department of Anatomical Pathology, Dr. Sardjito Hospital or of other hospitals. Paraffin tissue slides (3 mm-thick) were evaluated by immunohistochemistry using antibodies anti-VEGF-A (ABCAM cat. No. ab183100) for VEGF-A expression and anti-CD31 (BIOCARE MEDICALL, LLC, 4040 Pike Lane Concord, CA 94520 USA cat. No CM 347 A,C) for MVD after peroxidase activity blocking and heat antigen retrieval. The immunohistochemistry kit used in this experiment was Star Trek Universal HRP Detection System (STUHRP700 H, L10, BIOCARE). Non-reactive sites were blocked using background sniper. Primary antibody was applied, followed with the adding of secondary antibody (Trekkie Universal Link), and antibody binding was detected with diaminobenzidine (DAB) after the labeling with Trek Avidin HRP. Sections were counterstained with Mayer’s Hematoxylin. Tonsil samples were used as positive control for VEGF-A and CD31. These samples were also used as negative control by omitting the primary antibodies. Immunohistochemistry tests that demonstrated inappropriate staining for negative or positive controls were considered non-conclusive and the CRC samples in the same tests were not included in further analysis. The immunohistochemistry technique was performed at the Department of Anatomical Pathology and Department of Histology and Cell Biology, Faculty of Medicine, Universitas Gadjah Mada Yogyakarta. VEGF-A expression and MDV were independently assessed by two investigators who were blinded to the clinical data. Discordant findings were discussed and examined under a double-head microscope in order to determine a final assessment.
Evaluation of VEGF-A expression
The expressions were examined semi-quantitatively according to Martins et al., 2013, as follows, 0: 0% of immunoreactive cells; 1: <5% of immunoreactive cells; 2: 5–50% of immunoreactive cells; and 3: >50% of immunoreactive cells. The staining intensity was semi-qualitatively scored as 0: negative; 1: weak; 2: intermediate; and 3: strong. The final score for the immunoreaction was calculated as the sum of both parameters (number of immunoreactive cells and intensity), and grouped as negative [0], weak [0–2], moderate [2–4], and strong [4–6] (11). For statistical purposes, the negative and weak immunoreaction final scores were considered as low expression and strong immunoreaction final scores were considered as high expression.
Quantification of MVD
To determine the MVD a picture of “hot-spot” regions of the peritumoral area were made at 400× high power fields. The pictures were taken with microscope LSM-800 (Carl-ZEISS). Calculation of MVD was principally performed according to Bosari et al., 1992 (29), with certain modifications. Lumens or clusters formed by stained endothelial cells were considered as individual microvessels. Counting process excluded microvessels with a thick muscular wall. The slides were evaluated at 100× magnification to identify the “hot-spot” regions. The five most vascularized areas were selected and counted at 400× magnification. Three of the five highest vascularized regions were selected as the host-spot areas and calculated as the MVD per histological field, which was converted from the field of 400× magnification into 0.73 mm2. MVD indicated the average number of microvessels at the three most vascularized areas of 0.73 mm2. The MVD median (23.5) was used as the cut-off value for the MVD in the local cohort samples.
Statistical analysis
Statistical analysis was performed with the SPSS statistics 17 (Armonk, NY, USA). A Chi-squared test or Fisher’s exact test (2-sided) for noncontinuous variables and Mann-Whitney U or Kruskal-Wallis analysis for continuous variables were used to compare VEGF-A and MVD among the clinicopathological parameters. Survival was measured from the date of diagnosis until death or censored at the date of last follow-up. All patients were followed from diagnosis until death or until data were censored and the patient still considered to be alive. The OS curves were plotted using the Kaplan-Meier method and compared by the log-rank test. Prognostic variables of survival were analyzed by Cox proportional hazards regression. Significance was considered statistically different at P<0.05.
Results
Patient sample classification
We analyzed paraffin-embedded samples from tumors from 81 resected patients for CRC. Clinical and pathological features of all cases are displayed in Table 1. The median age at diagnosis was 56 years (range, 24–84 years). Population was dominated by males (46, 56.8%), individuals aged ≤56 years (42, 51.9%), cases with rectal location (52, 64.2%), cases with non-metastatic disease (47, 58.0%), tumors with well differentiated histology grade (44, 54.3%), tumors with T1–3 invasion (44, 54.3%), and tumors with unknown nodal status (47, 58.0%).
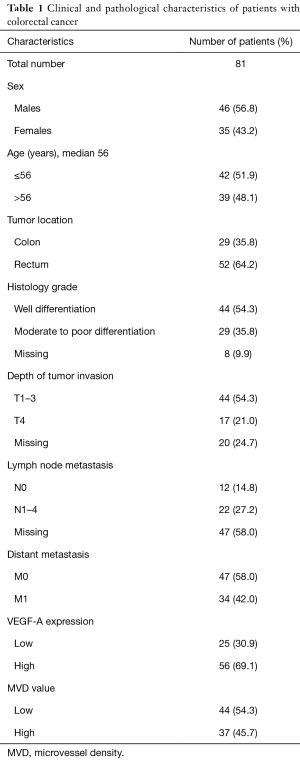
Full table
Immunohistochemical expression level of VEGF-A and MVD value across clinicopathologic variables
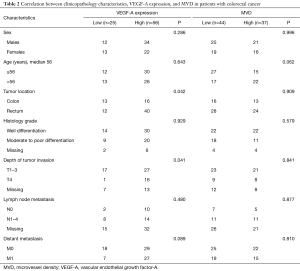
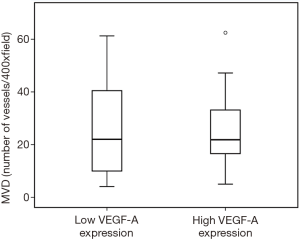
Figures 1-3 demonstrated immunohistochemical VEGF-A and MVD staining in patients with CRC in the present study. Low expression of VEFG-A was demonstrated by 25 (30.9%) subjects while high expression of VEGF-A was shown by 56 (69.1%) subjects. The median of MVD counts was 23.5 (range, 4.1–62.5). Low MVD value (≤23.5) was shown by 44 (54.3%) subjects while high MVD (>23.5) was demonstrated by 37 (45.7%) subjects. Analysis of clinicopathologic parameters and VEGF-A expression and MVD value are summarized in Table 2. VEGF-A expression was significantly higher in the rectum (P=0.042) and in T4 tumors (P=0.041), compared to their counterparts. There were no significant differences in the MVD according to all clinicopathologic characteristics, although it tended to be higher in older subjects compared to the younger cases (P=0.062). In addition, we determined any difference between low and high value of angiogenic markers. The distribution of MVD value in both groups of VEGF-A expression is shown in Figure 4. Analysis using Mann-Whitney U test showed that the median MVD count in the low VEGF-A expression and high VEGF-A expression was in a similar range (22.0±17.7 vs. 21.9±11.6; P=0.984).
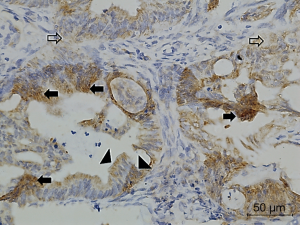
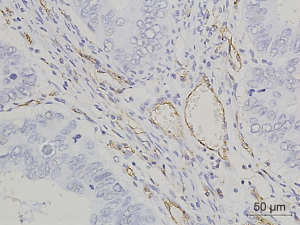
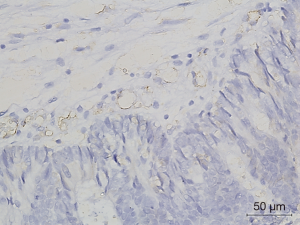
Full table
Survival analysis
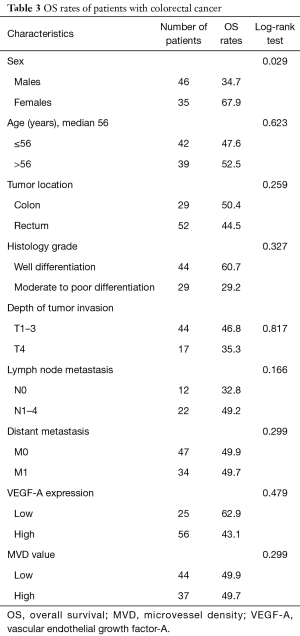
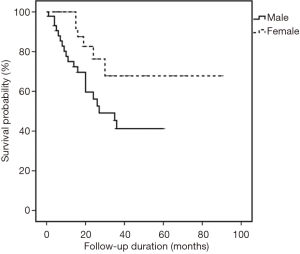
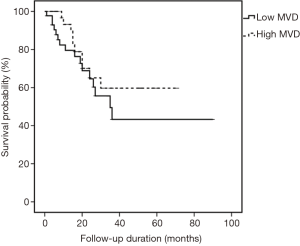
All cases were followed-up until September 2015. The median follow-up period was 18 months (range, 1–90 months). In the univariate analysis, sex was the only significant factor among all clinicopathological variables that predicted patients’ OS (P=0.029) (Table 3). Cox proportional hazard model revealed that males had a significantly increased probability of death compared to females (HR =2.641, 95% CI: 1.059–6.589; P=0.037) (Figure 5). Figures 6,7 showed no significant differences in the OS rates between the low and high VEGF-A expression groups (P=0.479), or between the low and high MVD groups (P=0.299).
Full table

Discussion
Angiogenesis is a crucial step in tumor development and metastasis (2,4). Tumor angiogenic activity may reflect an aggressive behaviour of a malignancy (6). Among other angiogenesis-stimulating proteins, VEGF-A has been long observed as the most potent angiogenic factor and is commonly associated with increased MDV and unfavourable clinicopathological parameters and outcome (8,18). This retrospective study investigated the expression of VEGF-A and determined the count of MVD in 81 local patients with CRC. High levels of VEGF-A expression and MVD value were demonstrated by 56 (69.1%) and 37 (45.7%) cases, respectively. Ganggaiswari et al. reported a higher proportion (71.8%) of cases with strong expression of VEGF-A level from another cancer centre in Indonesia. However, the report of Ganggaiswari and ours might not fully represent the proportion of VEGF-A level in all CRC cases in the local regions since both studies collected only cases with available pathology samples. MVD value has never been analyzed in Indonesian CRC patients. We have previously tested MVD in selected cases with nasopharyngeal cancer and our report showed a 50% proportion of high MVD in those patients (30).
Among all clinicopathological variables, significant correlations were seen between high VEGF-A expression with rectal location and T4 tumour invasion, supporting previous findings (11,17,25,31). Bendardaf et al. showed that VEGF positivity and intensity were confined to the left-sided or distal region. The association of higher levels of VEGF-A expression with distal tumours may be due to the different genetic pathway that the left and right-sided CRCs are considered to take. This emphasized clinical impact of the present study on the existence of biology differences between right and left colonic location that could favour malignant transformation through different molecular pathways (31,32). Hanrahan et al. also indicated an association between VEGF-A strong expression and T4 depth invasion that contribute to progression, invasion, and metastasis and unfavorable survival and prognosis in CRC (33). Furthermore, our study showed a tendency of higher proportion of cases with metastatic disease with high VEGF-A expression, as found by others (18,34).
Although in contrast to the vast majority of previous observations, our study did not demonstrate any significant relationship between MVD value with all clinicopathological variables. Similar results were also shown by other reports (15,16,24). Interestingly, many studies that showed significant correlation of MVD count with clinicopathological parameters used antibody against anti CD34 or von Willebrand (13,14,25,35,36), although some reports also used anti CD31 antibody as ours, or anti CD105 antibody (37). Different methods may influence the results and the variability needs to be further investigated. Our study also showed that older patients had a tendency to have a higher frequency of high MVD value, as investigated by Sundov et al. (35) although it used age of >60 years as a cut off value for analysis.
Evidence from multiple studies implicates VEGF and MVD value as being prognostic indicators in CRC as high expression and value associated with poor survival. Similarly, meta-analysis reports from Des Guetz and Wang et al. confirmed the association with poor OS in CRC (8,18). Our study, however, did not support their prognostic significance in the local CRC patients. The finding might represent the nature of our local patients or was a consequence of relatively small sample size in the present study. In addition, our previous report analyzing plasma VEGF-A and MVD count in nasopharyngeal cancer showed shorter survival of patients with high expression of VEGF-A and MVD count compared to those with low expression and count (30).
Our result demonstrating that male sex had an increased probability of mortality was supported by a large prospective study from Malaysia that was done in a tertiary hospital as ours. It demonstrated that male sex was one of independent predictors of poor 5-year survival (HR =1.41; 95 CI: 1.12–1.76) (38). In addition, males in our study cohort had a higher frequency of high VEGF-A staining intensity compared to females, despite statistically being a non-significant difference. This indicated a linear association of high VEGF-A expression with shorter survival. However, the true prognostication role of sex in our local cases needs to be further investigated in a bigger patient population.
Conclusions
In the local cohort, VEGF-A expression had a clinical significance according to tumor location and depth, while MVD value had no differences accross all clinicopathological variables. VEGF-A expression and MVD value did not have any impact on the patients’ OS, but sex did.
Acknowledgements
The authors are grateful to Ibnu Purwanto, Kartika Widayati Taroeno-Hariadi, and Mardiah Suci Hardianti who provided and cared for study patients, and Via Wahyu Ferianti, Guntara Khuzairi, Wiwit Setyowati, Dewi Sulistyawati, Agustine, and Sri Wahyuni for technical assistance. The authors also thanks Adrian Coen and the staff of “Klinik Bahasa”, Office for Research and Publication, Faculty of Medicine, Universitas Gadjah Mada, Indonesia, for proofreading the text.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of Faculty of Medicine Universitas Gadjah Mada/Dr Sardjito Hospital Yogyakarta (reference: KE/FK/209/EC). Informed consent was obtained from study subjects.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990;82:4-6. [Crossref] [PubMed]
- Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992;267:10931-4. [PubMed]
- Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 1995;333:1757-63. [Crossref] [PubMed]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86:353-64. [Crossref] [PubMed]
- Giatromanolaki A, Sivridis E, Koukourakis MI. Angiogenesis in colorectal cancer: prognostic and therapeutic implications. Am J Clin Oncol 2006;29:408-17. [Crossref] [PubMed]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997;18:4-25. [Crossref] [PubMed]
- Des Guetz G, Uzzan B, Nicolas P, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 2006;94:1823-32. [Crossref] [PubMed]
- Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis-correlation in invasive breast carcinoma. N Engl J Med 1991;324:1-8. [Crossref] [PubMed]
- Wen L, Wang R, Lu X, et al. Expression and clinical significance of vascular endothelial growth factor and fms-related tyrosine kinase 1 in colorectal cancer. Oncol Lett 2015;9:2414-8. [Crossref] [PubMed]
- Martins SF, Garcia EA, Luz MA, et al. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genomics Proteomics 2013;10:55-67. [PubMed]
- Ganggaiswari A, Kresno SB, Krisnuhoni E. VEGF expression and desmoplastic reaction as potential progressive factors in young patients with colorectal cancer. Acta Med Indones 2010;42:6-11. [PubMed]
- Goldiş DS, Sferdian MF, Tarţă C, et al. Comparative analysis of microvessel density quantified through the immunohistochemistry expression of CD34 and CD105 in rectal cancer. Rom J Morphol Embryol 2015;56:419-24. [PubMed]
- Zheng S, Han MY, Xiao ZX, et al. Clinical significance of vascular endothelial growth factor expression and neovascularization in colorectal carcinoma. World J Gastroenterol 2003;9:1227-30. [Crossref] [PubMed]
- Gao J, Knutsen A, Arbman G, et al. Clinical and biological significance of angiogenesis and lymphangiogenesis in colorectal cancer. Dig Liver Dis 2009;41:116-22. [Crossref] [PubMed]
- Svagzdys S, Lesauskaite V, Pavalkis D, et al. Microvessel density as new prognostic marker after radiotherapy in rectal cancer. BMC Cancer 2009;9:95. [Crossref] [PubMed]
- Zong S, Li H, Shi Q, et al. Prognostic significance of VEGF-C immunohistochemical expression in colorectal cancer: A meta-analysis. Clin Chim Acta 2016;458:106-14. [Crossref] [PubMed]
- Wang Y, Yao X, Ge J, et al. Can vascular endothelial growth factor and microvessel density be used as prognostic biomarkers for colorectal cancer? A systematic review and meta-analysis. ScientificWorldJournal 2014;2014:102736. [PubMed]
- Bendardaf R, El-Serafi A, Syrjanen K, et al. The effect of vascular endothelial growth factor-1 expression on survival of advanced colorectal cancer patients. Libyan J Med 2017;12:1290741. [Crossref] [PubMed]
- Araújo RF Jr, Lira GA, Vilaça JA, et al. Prognostic and diagnostic implications of MMP-2, MMP-9, and VEGF-alpha expressions in colorectal cancer. Pathol Res Pract 2015;211:71-7. [Crossref] [PubMed]
- Cho T, Shiozawa E, Urushibara F, et al. The role of microvessel density, lymph node metastasis, and tumor size as prognostic factors of distant metastasis in colorectal cancer. Oncol Lett 2017;13:4327-33. [Crossref] [PubMed]
- White JD, Hewett PW, Kosuge D, et al. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res 2002;62:1669-75. [PubMed]
- Gulubova M, Vlaykova T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol 2009;24:1265-75. [Crossref] [PubMed]
- Martinovic Z, Kovac D, Martinovic M. Prognostic Significance of Microvessel Density Determining by Endoglin in Stage II Rectal Carcinoma: A Retrospective Analysis. Gastroenterol Res Pract 2015;2015:504179. [Crossref] [PubMed]
- Nakasaki T, Wada H, Shigemori C, et al. Expression of tissue factor and vascular endothelial growth factor is associated with angiogenesis in colorectal cancer. Am J Hematol 2002;69:247-54. [Crossref] [PubMed]
- Perrone G, Vincenzi B, Santini D, et al. Correlation of p53 and bcl-2 expression with vascular endothelial growth factor (VEGF), microvessel density (MVD) and clinico-pathological features in colon cancer. Cancer Lett 2004;208:227-34. [Crossref] [PubMed]
- Barresi V, Di Gregorio C, Regiani-Bonetti L, et al. Stage I colorectal carcinoma: VEGF immunohistochemical expression, microvessel density, and their correlation with clinical outcome. Virchows Arch 2010;457:11-9. [Crossref] [PubMed]
- Anannamcharoen S, Nimmanon T. Study of the vascular endothelial growth factor (VEGF) expression and microvascular density (MVD) in primary colorectal cancer specimens. J Med Assoc Thai 2012;95:1041-7. [PubMed]
- Bosari S, Lee AK, DeLellis RA, et al. Microvessel quantitation and prognosis in invasive breast carcinoma. Hum Pathol 1992;23:755-61. [Crossref] [PubMed]
- Kurnianda J, Hardianti MS. Elevation of vascular endothelial growth factor in Indonesian advanced stage nasopharyngeal carcinoma. Kobe J Med Sci 2009;55:E36-44. [PubMed]
- Bendardaf R, Buhmeida A, Hilska M, et al. VEGF-1 expression in colorectal cancer is associated with disease localization, stage, and long-term disease-specific survival. Anticancer Res 2008;28:3865-70. [PubMed]
- Bleeker WA, Hayes VM, Karrenbeld A, et al. Impact of KRAS and TP53 mutations on survival in patients with left- and right-sided Dukes' C colon cancer. Am J Gastroenterol 2000;95:2953-7. [Crossref] [PubMed]
- Hanrahan V, Currie MJ, Gunningham SP, et al. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J Pathol 2003;200:183-94. [Crossref] [PubMed]
- Kwak Y, Lee HE, Kim WH, et al. The clinical implication of cancer-associated microvasculature and fibroblast in advanced colorectal cancer patients with synchronous or metachronous metastases. PLoS One 2014;9:e91811. [Crossref] [PubMed]
- Sundov Z, Tomic S, Alfirevic S, et al. Prognostic value of MVD, LVD and vascular invasion in lymph node-negative colon cancer. Hepatogastroenterology 2013;60:432-8. [PubMed]
- Dumitrescu TV, Uscatu CD, Mogoanta SS, et al. Preliminary study of correlations between the intratumoral microvessel density and the morphological profile of colorectal carcinoma. Rom J Morphol Embryol 2015;56:679-89. [PubMed]
- Gurzu S, Jung J, Azamfirei L, et al. The angiogenesis in colorectal carcinomas with and without lymph node metastases. Rom J Morphol Embryol 2008;49:149-52. [PubMed]
- Magaji BA, Moy FM, Roslani AC, et al. Survival rates and predictors of survival among colorectal cancer patients in a Malaysian tertiary hospital. BMC Cancer 2017;17:339. [Crossref] [PubMed]


