CT-based assessment of visceral adiposity and outcomes for esophageal adenocarcinoma
Introduction
Accounting for an estimated 16,980 new cases and 15,590 deaths in the United States in 2015, esophageal cancer has been identified as the eighth most commonly diagnosed cancer and the sixth leading cause of cancer-related deaths in the world (1-4). While the rate of squamous cell carcinoma (the most common histological subtype worldwide) is decreasing in the West, adenocarcinoma is becoming increasingly prevalent in northern Europe and North America (5-8). Esophageal adenocarcinoma (EA) has the fastest increasing incidence of all malignancies in the United States, with a growth rate of over 500% in the past 30 years (5,9).
This rise in EA has been linked to the recent increase in obesity (7,10). Epidemiological evidence has identified a positive correlation between various measures of obesity and increasing risk for EA, with one recent meta-analysis showing a 3X increase risk associated with body mass index (BMI) ≥30 kg/m2 (7,11,12). Obesity, moreover, is thought to be a prognostic factor in addition to a risk factor for EA (13). Body fat is traditionally distributed into two main compartments with different metabolic characteristics: subcutaneous fat (SFA) (peripheral) and visceral fat (VFA) (abdominal) with total fat (TFA) as a combination of SFA and VFA. More recently published data accounting for body fat distribution suggests that quantification of adiposity, especially visceral adiposity, rather than BMI, is a more accurate measure for evaluating the associated risk and prognosis (7,14). This finding has helped explain the epidemiological observation that men (who accumulate fat primarily in their abdominal area) have a significantly higher incidence of EA than women (who first accumulate fat in their hips and thighs) (15). Incidence of EA in men is 7 times that found in women (16). Studies that compare the impact of visceral fat with subcutaneous fat (fat located just beneath the skin) and total fat adiposity on EA and other GI malignancies have found that VFA displays a strong correlation to risk and incidence, whereas SFA and TFA are not strongly correlated.
Standard treatment of resectable EA is esophagectomy (17). Authors have examined the association between BMI and oncologic outcomes as well as peri-operative complications. Respiratory complications, anastomotic leak, and worsening of disease-specific survival, and overall survival have been reported in obese patients who underwent surgery for EA (13,18,19). However, studies on postoperative survival probability associated with measures of adiposity have been less clear. We evaluated preoperative adiposity as a prognostic factor for postoperative outcomes in patients with EA who have undergone esophagectomies.
Methods
Patients
Our single institution study included 126 patients with EA who underwent esophagectomy from 2008–2012. They were retrospectively identified from a GI esophageal database (Institutional Review Board-approved esophagectomy database). Physicians reviewed and recorded all charts on standardized abstraction forms. To be included all patients had CT scans to determine adiposity, a diagnosis of adenocarcinoma, and underwent surgical resection. Patients with BMI <20 were excluded.
Determination of adiposity
Using the preoperative CT scan, the VFA, SFA, and TFA were calculated. Each was contoured on a Siemens Leonardo workstation at the level of the iliac crest (L4/5). The Hounsfield threshold was −30 to −130.
Oncologic assessment
All patients in our analysis underwent preoperative clinical staging with physical exams, endoscopy including endoscopic ultrasound (EUS), computed tomography (CT) scans, and positron emission tomography (PET) scans. Pathologists with specialty training in GI malignancies confirmed all pathology. Staging, lymph nodes, tumor size, histological grade, response to therapy, recurrence, and residual tumor were determined using postoperative pathology reports. Overall survival was defined as the time from date of surgery to date of death, while disease-free survival was defined as time from date of surgery to the date of discovery of recurrence. Overall survival was evaluated using medical records and social security death index.
Statistical analysis
Univariate analysis was used to examine whether VFA or VFA/SFA ratio were associated with pre-treatment stage, number of involved lymph nodes, proportion of patients treated with neoadjuvant therapy, tumor size, operation time, type of surgery, histological grade, gender, age, pathological response to therapy, or radial margins and residual tumor. Comparison of characteristics between groups was evaluated using Chi-squared analysis. Discrete variables between groups were compared using Fischer’s exact tests. Multivariate analysis (MVA) was used to assess VFA, TFA, SFA, and VFA/SFA as prognostic factors, and was performed using the Cox proportion hazard regression model. The Kaplan-Meier method and log-rank analysis were used to analyze disease-free survival and overall survival. Statistical significance was defined as P≤0.05. All analyses were performed using the STATA IC (Stata Statistical Software, Release 10.0; Strata Corp., College Station, TX, USA).
Results
We identified 126 patients who underwent esophagectomy for adenocarcinoma who met the inclusion criteria for this study. The medians for VFA, TFA, SFA, and VFA/SFA ratio were 182, 491.7, 281, and 0.655 cm2, respectively. Univariate analyses of patient characteristics with VFA and with VFA/SFA ratio medians are presented in Tables 1 and 2, respectively. VFA ≥182 cm2 was associated with fewer lymph nodes harvested (P=0.047), larger tumor size (P=0.016), male gender (P=0.042), and longer operating times (P=0.032). There were no statistically significant differences in clinical stage (T stage, P=0.687; N stage, P=0.895), proportion of patients treated with neoadjuvant therapy (P=0.563), type of surgery performed (P=0.342), histological grade (P=0.452), pathologic response to therapy (P=0.848), recurrence (P=0.660), R0 resections (P=0.080), or mean age (P=0.150) between groups.
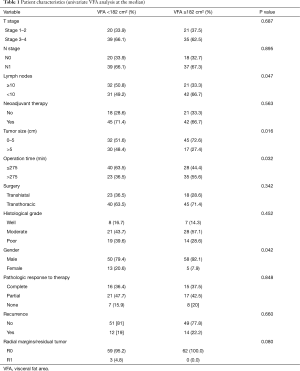
Full table
Full table
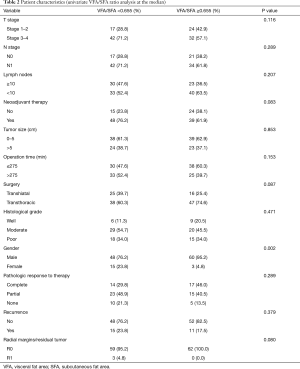
The only significant differences found by VFA/SFA ratio univariate analysis were gender (P=0.002) and mean age (P=0.002). Clinical stage (T stage, P=0.116; N stage, P=0.289), number of lymph nodes (P=0.207), proportion of patients treated with neoadjuvant therapy (P=0.083), tumor size (P=0.853), operating time (P=0.153), type of surgery performed (P=0.087), histological grade (P=0.471), pathologic response to therapy (P=0.289), recurrence (P=0.379), and resection level (P=0.080), were comparable between groups.
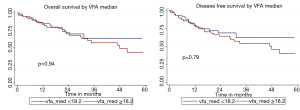
Probabilities for overall survival and disease-free survival were determined in relation to VFA, SFA, TFA, and VFA/SFA ratio. For each measure of adiposity, patients were analyzed for OS and DFS. There were no statistically significant differences in DFS or OS between groups for any measure of adiposity. Figure S1 demonstrates comparisons of OS and of DFS between groups above and below the VFA median. The 5-year and median OS for patients with VFA <182 and ≥182 were 63.63% and 67.6 months, and 43.77% and 47.9 months, respectively (P=0.94). The respective 5-year and median DFS were 62.48% and 60.4 months, and 40.22% and 47.9 months (P=0.79). Figure S2 displays overall and disease-free survival analysis for TFA. The 5-year and median OS for groups with TFA <491.7 and ≥491.7 were 68.12% and 67.6 months, and 42.47% and 47.9 months, respectively (P=0.78). The 5-year and median DFS for patients below and above TFA median were 64.55% and 67.6 months, and 41.54% and 47.9 months, respectively (P=0.75).
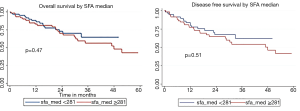
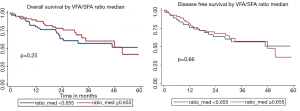
Comparisons of OS and DFS between groups above and below SFA median are shown in Figure S3. The 5-year and median OS for groups with SFA <281 and ≥281 were 64.43% and 67.6 months, and 43.00% and 47.9 months, respectively (P=0.47). The 5-year and median DFS below and above the median were 61.89% and 67.6 months, and 41.47% and 47.9 months, respectively (P=0.51). Figure S4 displays OS and DFS for VFA/SFA ratio survival analysis. The 5-year and median OS for patients with VFA/SFA <0.655 and ≥0.655 were 51.32% and 63.9 months, and 41.62% and 51.57 months, respectively (P=0.25). The 5-year and median DFS below and above VFA/SFA median were 50.98% and 60.4 months, and 35.88% and 47.9 months, respectively (P=0.66).
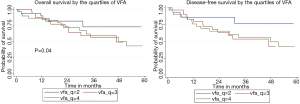
We further classified patients into quartiles rather than median in an effort to identify any possible confounders from the lowest VFA/BMI categories which could possibly skew outcomes. We analyzed OS and DFS based upon quartiles for VFA. Quartile 1 was defined as 37.7 cm2 ≥ VFA ≥117.2 cm2; quartile 2 was defined as 120≥ VFA ≥181.8; quartile 3 was defined as 182.2≥ VFA ≥254.2; and quartile 4 was defined as 254.9≥ VFA ≥700.8. BMI equivalents for each quartile are shown in Table 3. Excluding quartile one, we found a significant decrease overall survival and disease-free survival with increasing VFA from quartile two to quartile three to quartile four. Figure 1 demonstrates comparisons of OS and of DFS by VFA quartiles (quartiles 2, 3, and 4). The 5-year and median OS for quartile one was 52.12% and 67.6 months; for DFS, 45.44% and 22.8 months, respectively. The 5-year and median OS and DFS for quartile two was 72.68% and 89.3 months, and 76.39% and 60.4 months, respectively. The 5-year and median OS for quartile three was 49.18% and 46.9 months; the 5-year and median DFS was 42.65% and 46.9 months, respectively. The 5-year and median OS for quartile four was 43.20% and 51.57 months; the 5-year and median DFS was 42.24% and 47.9 months, respectively.

Full table
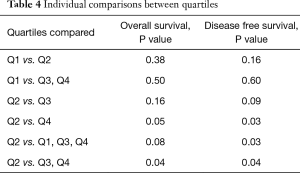
Individual comparisons between quartiles are shown in Table 4. Quartile 2 demonstrated a significant improvement in overall survival when compared to quartile 3 and 4 combined, P=0.04 and when compared to quartile 4 individually, P=0.05. Additionally, patient’s in quartile 2 had improvement in disease free survival when compared to quartiles 1, 3, and 4 combined, P=0.03, and quartile 4 individually, P=0.03. Patients in the lowest VFA/BMI quartile exhibited similar worse overall and disease free survival as those patients in the higher VFA quartiles, P=0.5, and 0.6 respectively.
Full table
Discussion
We found no significant correlation between either SFA or TFA and overall survival. Survival analysis also demonstrated no association between either SFA or TFA and disease-free survival. VFA/SFA ratio was not significantly associated with either OS or DFS. However, upon investigating the impact of VFA based on quartiles, we found a significant decrease in OS and DFS with increasing VFA. Quartile one consists of patients with the lowest VFA, and most likely represents patients who could have been malnourished or have less reserve to tolerate surgery. Quartile two was comprised of patients with mildly elevated VFAs, but the majority were in the correlative BMI 25–29 category; survival outcomes progressively worsened as VFA increased from quartile two to quartile three and quartile four.
The global incidence of obesity is rapidly increasing, with an estimated 1.46 billion overweight adults in 2008, 502 of which million were obese (20,21). It is projected that by 2030 there will be 65 million more obese adults in US and 11 million more in the UK (21). The mean overall survival for people diagnosed with EA is merely 12% (22). While epidemiologic evidence has clearly shown the connection between various measures of obesity and increased risk for EA, the association between obesity (especially measured as visceral adiposity) and prognosis has been explored only recently (23). The role of adiposity in postoperative survival is becoming increasingly important in the context of these obesity trends.
While existing data have demonstrated an association between treatment outcomes and degree of adiposity, our study found that only VFA was significantly associated with OS or DFS. TFA, SFA, and VFA/SFA were not prognostic on MVA. While VFA >182 cm2 was associated with larger tumors, there were also fewer lymph nodes harvested in this group and no association with lymph node positivity.
The mechanistic link between VFA and EA is twofold. Excess VFA promotes gastroesophageal reflux (14). This may lead to the development of Barrett’s esophagus (BE), a pre-neoplastic lesion and a known precursor to EA (24). One case-control study demonstrated a 4.1-fold increased risk for BE associated with high waist-to-hip ratio, ≥0.90 for men and ≥0.85 for women (P=0.003). However, adjustment for BMI showed no increase in risk (25). In addition, visceral fat is metabolically active, secreting a number of adipokines, cytokines, and growth factors that may cause the esophageal inflammation leading to carcinogenesis (12,14).
Moreover, VFA has been shown to be a prognostic factor in other gastrointestinal malignancies including: colorectal, gastric, and pancreatic cancers. The authors of a single center Japanese study found that visceral obesity based on waist circumference was a significant risk factor for systematic complications following laparoscopic surgery for colorectal cancer. However, BMI had no independent impact on patient outcome (26). Similarly Seki et al., in a retrospective study investigating the impact of visceral fat on operation time, reported that VFA/BSA may be a more useful index than BMI in predicting technical difficulty of performing a laparoscopic resection of rectosigmoid carcinoma (27). In a study investigating the prognostic significance of visceral obesity and BMI in 161 resectable colorectal cancer patients, Moon et al. reported that increased visceral adiposity was a significant predictor of disease-free survival in patients with resectable colorectal cancer (28).
A study from the Cancer Institute Hospital in Tokyo on the relationship between fat area and early post-operative outcomes in patients undergoing gastrectomy found that patients with a high VFA are more likely to develop an intra-abdominal infection following surgery. However, SFA and BMI did not correlate with worsened surgical outcomes (29). Similarly, Yoshikawa et al. found that the area of visceral fat tissue was more useful than BMI in predicting the risks of laparoscopy assisted gastrectomy and postoperative complications (30). Using preoperative CT imaging to measure intra-abdominal (visceral) fat, Balentine et al. reported found that increased visceral fat correlates to a worse prognosis in pancreatic cancer (31).
Obesity has been found to be positively correlated to development of EA (32). More recent studies have found the VFA rather than BMI is responsible for the associated increase in risk and incidence for EA (33). However, the correlation between measures of obesity and survival outcomes in patients undergoing resection for EA is less clear. We have previously demonstrated that BMI was neither associated with surgical complications nor survival in patients with EA treated with neoadjuvant chemo-radiation therapy (5).
Given that VFA has been found to be a greater predictor than BMI of outcomes in patients with other GI malignancies, our study offers a more accurate analysis of the association between body fat and outcomes in EA. Patients in the higher VFA quartiles are at greatest risk for recurrence and decreased survival. Other measurements such as TFA and SFA had no correlation between tumor recurrence or survival. Further studies with a larger number of patients should be conducted to further explore the impact of visceral fat adiposity on outcomes in patients with EA.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Moffitt Cancer Center (#105286Z).
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893-907. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [Crossref] [PubMed]
- Shridhar R, Hayman T, Hoffe S, et al. Body Mass Index and Survival in Esophageal Adenocarcinoma Treated with Chemoradiotherapy Followed by Esophagectomy. J Gastrointest Surg 2012;16:1296-302. [Crossref] [PubMed]
- Simard EP, Ward EM, Siegel R, et al. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 2012;62:118-28. [Crossref] [PubMed]
- Steffen A, Schulze MB, Pischon T, et al. Anthropometry and Esophageal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev 2009;18:2079-89. [Crossref] [PubMed]
- Bosetti C, Levi F, Ferlay J, et al. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer 2008;122:1118-29. [Crossref] [PubMed]
- Kresty LA, Exum A, Zeyzus-Johns B. Berries in the Prevention of Esophageal Adenocarcinoma. In: Seeram NP, Stoner GD. editors. Energy Balance and Gastrointestinal Cancer. New York: Springer, 2012;101-15.
- Leidner R, Chak A. Obesity and the Pathogenesis of Barrett’s Esophagus. In: Markowitz S, Berger N. editors. Energy Balance and Gastrointestinal Cancer. New York: Springer, 2012;77-92.
- Lagergren J. Influence of obesity on the risk of esophageal disorders. Nat Rev Gastroenterol Hepatol 2011;8:340-7. [Crossref] [PubMed]
- Doyle SL, Donohoe CL, Finn SP, et al. IGF-1 and its receptor in esophageal cancer: association with adenocarcinoma and visceral obesity. Am J Gastroenterol 2012;107:196-204. [Crossref] [PubMed]
- Yoon HH, Lewis MA, Shi Q, et al. Prognostic impact of body mass index stratified by smoking status in patients with esophageal adenocarcinoma. J Clin Oncol 2011;29:4561-7. [Crossref] [PubMed]
- Beddy P, Howard J, McMahon C, et al. Association of visceral adiposity with oesophageal and junctional adenocarcinomas. Br J Surg 2010;97:1028-34. [Crossref] [PubMed]
- Quigley EM, Jacobson BC, Lenglinger J, et al. Barrett's esophagus: clinical features, obesity, and imaging. Ann N Y Acad Sci 2011;1232:36-52. [Crossref] [PubMed]
- Vial M, Grande L, Pera M. Epidemiology of adenocarcinoma of the esophagus, gastric cardia, and upper gastric third. Recent Results Cancer Res 2010;182:1-17. [PubMed]
- Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. The Am J Surg 2003;185:538-43. [Crossref] [PubMed]
- Healy LA, Ryan AM, Gopinath B, et al. Impact of obesity on outcomes in the management of localized adenocarcinoma of the esophagus and esophagogastric junction. J Thorac Cardiovasc Surg 2007;134:1284-91. [Crossref] [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [Crossref] [PubMed]
- Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet 2011;378:804-14. [Crossref] [PubMed]
- Wang YC, McPherson K, Marsh T, et al. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011;378:815-25. [Crossref] [PubMed]
- Herszényi L TZ. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci 2010;14:249-58. [PubMed]
- Irwin ML, American College of Sports Medicine. editors. ACSM's Guide to Exercise and Cancer Survivorship. Human Kinetics, 2012.
- van de Winkel A, Massl R, Kuipers EJ, et al. Digestive disease week 2011: highlights of clinical and preclinical research on Barrett's esophagus and associated esophageal adenocarcinoma. Dis Esophagus 2013;26:130-40. [Crossref] [PubMed]
- Reid BJ, Li X, Galipeau PC, et al. Barrett's oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer 2010;10:87-101. [Crossref] [PubMed]
- Nitori N, Hasegawa H, Ishii Y, et al. Impact of visceral obesity on short-term outcome after laparoscopic surgery for colorectal cancer: a single Japanese center study. Surg Laparosc Endosc Percutan Tech 2009;19:324-7. [Crossref] [PubMed]
- Seki Y, Ohue M, Sekimoto M, et al. Evaluation of the technical difficulty performing laparoscopic resection of a rectosigmoid carcinoma: visceral fat reflects technical difficulty more accurately than body mass index. Surg Endosc 2007;21:929-34. [Crossref] [PubMed]
- Moon HG, Ju YT, Jeong CY, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Ann Surg Oncol 2008;15:1918-22. [Crossref] [PubMed]
- Tokunaga M, Hiki N, Fukunaga T, et al. Effect of individual fat areas on early surgical outcomes after open gastrectomy for gastric cancer. Br J Surg 2009;96:496-500. [Crossref] [PubMed]
- Yoshikawa K, Shimada M, Kurita N, et al. Visceral fat area is superior to body mass index as a predictive factor for risk with laparoscopy-assisted gastrectomy for gastric cancer. Surg Endosc 2011;25:3825-30. [Crossref] [PubMed]
- Balentine CJ, Enriquez J, Fisher W, et al. Intra-abdominal fat predicts survival in pancreatic cancer. J Gastrointest Surg 2010;14:1832-7. [PubMed]
- Ryan AM, Duong M, Healy L, et al. Obesity, metabolic syndrome and esophageal adenocarcinoma: Epidemiology, etiology and new targets. Cancer Epidemiol 2011;35:309-19. [Crossref] [PubMed]
- Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev 2008;17:352-8. [Crossref] [PubMed]



