Is exposure to Agent Orange a risk factor for hepatocellular cancer?—A single-center retrospective study in the U.S. veteran population
Introduction
Approximately 80% to 90% of hepatocellular cancer (HCC) is associated with cirrhosis related to viral hepatitis (1). Hepatitis C virus (HCV) is prevalent in 2% of the world’s population, varying by region, and approximately 15% to 35% of those with HCV related cirrhosis will develop HCC (2). The incidence of HCC is 2% to 6% per year in patients with cirrhosis and HCV (3). With the burden of cirrhosis related to HCV increasing across the globe, and consequently, increasing the incidence of HCC, the identification of risk factors for HCC has become essential. The Veterans Health Administration (VHA) is the single largest HCV care provider in the U.S., with a seroprevalence rate of 5.4% and over 170,000 veterans with confirmed chronic hepatitis C (CHC) (4). The majority of these veterans were born between 1945 and 1965 and likely infected between 1970 and 1999 (4,5). However, about 20% of patients with HCV in Veterans Administration care are undiagnosed (4). Over the past 10 years, the number of HCV infected veterans with cirrhosis has tripled to over 25,000, while over the same time period the cumulative number of HCV-infected veterans diagnosed with HCC has increased ten-fold (6).
The increasing incidence of HCC is due to longer survival of patients with cirrhosis, improvements in screening and diagnosis, and greater burden of cirrhosis in the population born between 1945 and 1965, a group having the highest prevalence of HCV (7). Other factors implicated in the development of HCC in patients with HCV are older age, male gender, Hispanic race, smoking, high body mass index (BMI) (8-11) and comorbidities of alcoholic liver disease, diabetes mellitus, and portal hypertension (8-10,12-14). Viral related factors associated with HCC include viral genotype 3, genotype 1b, and high HCV RNA level in the blood (15-17). Patients who have been treated and attained sustained virological response (SVR) have a much lower incidence of HCC and all-cause mortality than patients who do not attain SVR (18,19).
In a South Korean study exposure to Agent Orange, a herbicide that was used in chemical warfare during the Vietnam War between 1961 and 1971, was implicated as a possible risk factor for liver cancer and other cancers (20). To the best of our knowledge, there has been no study in U.S. veterans with HCV and cirrhosis that has evaluated exposure to Agent Orange as a risk factor for HCC.
We conducted a retrospective study of U.S. military veterans diagnosed with HCV and cirrhosis over a period of 14 years to evaluate all the reported risk factors for HCC including exposure to Agent Orange.
Methods
This study was conducted at the Veterans Affairs Medical Center (VAMC) in Dayton, Ohio, a tertiary care referral center with an established gastroenterology outpatient practice as well as dedicated inpatient gastroenterology services. The Institutional Review Board at the Dayton VAMC and Wright State University approved the study. We obtained data from the Dayton VAMC’s HCV Clinical Case Registry (CCR), which contains health information for all known HCV infected patients. A list of patients carrying a diagnosis of hepatitis C between January 2000 and December 2013 at the Dayton VAMC was obtained from the registry. Patients with cirrhosis and confirmed hepatitis C were included in the study. Criteria for confirmation of HCV included positive qualitative, quantitative, and/or detectible viral genotype results. Patients with cirrhosis were identified using ICD-9 codes for cirrhosis (571.2, 571.5, 571.6), thrombocytopenia (287.5), ascites (789.5), hepatic encephalopathy (572.2), hepatorenal syndrome (572.4), and esophageal varices (456.2). Cirrhosis was considered a cause of thrombocytopenia if no other identifiable cause was found on review of records and was persistent for at least two consecutive years with platelet count of <150 t/mm. We excluded patients who did not have confirmation of HCV infection.
Patient data were obtained by reviewing the computerized patient record system (CPRS) as well as the VA intranet records. Follow-up time was censored at death, or the date of the last medical encounter. Cases of HCC were identified from the cohort that had confirmed HCV and cirrhosis, using ICD-9 code 155.0. We collected data on several potential risk factors for HCC including age at the time of HCV diagnosis, gender, race, viral genotype including the subtype for type 1 HCV, highest viral load recorded, treatment history, hepatitis B surface antigen status, diabetes mellitus, current or prior alcohol addiction, current or prior smoking addiction, and exposure to Agent Orange. Patients with alcohol addiction were identified using ICD-9 codes 305, 305.3, 303.9 and prior hospitalizations for alcohol addiction or withdrawal. Smoking addiction was identified using codes for tobacco and nicotine abuse disorders, ICD-9 code 305.1. We defined antiviral treatment as receiving at least 3 months of Interferon, ribavirin, and/or direct acting antiviral agents. Treatment history of HCV was further divided into no treatment, prior treatment with SVR, and prior treatment without SVR. Patients who were prior null responders, partial responders, or relapsers were considered as not achieving SVR. SVR was defined as negative viral load 6 months after completion of treatment. Other baseline characteristics for patients with HCC included Childs Pugh Turcotte score, alpha fetoprotein (AFP) level at diagnosis, and BMI 3 years prior to diagnosis of HCC (to avoid confounding factors such as weight loss from malignancy or weight gain from ascites).
Statistical analysis
Means and standard deviations are reported for continuous variables, and counts and percentages for categorical variables. The independent samples Mann-Whitney test was used for comparisons involving two groups and a second variable measured on a continuous scale.
The chi square test or Fisher’s exact test was used to compare two categorical variables. Multivariable logistic regression (MLR) was used to identify independent risk factors of HCC. Consistent with conventional practice, variables at P≤0.10 on univariate analysis were included in the MLR equation. Inferences were made at the 0.05 level of significance with no corrections for multiple comparisons. Analyses were conducted using IBM SPSS Statistics 22.0 (IBM, Armonk, NY, USA).
Results
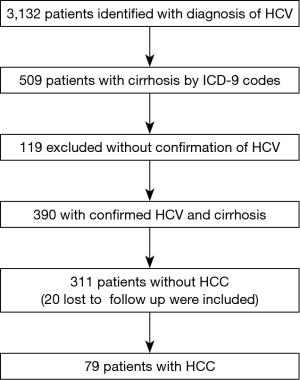
A total of 3,132 veterans were identified with a diagnosis of HCV from the National Hepatitis C Registry for the Veterans Administration between January 2000 and December 2013 who had an encounter at the Dayton VA Medical Center, of which 509 patients had cirrhosis (16.3%).
Of the 509 patients with cirrhosis, 390 patients had confirmed hepatitis C (76.6%). These 390 patients with both cirrhosis and confirmed hepatitis C were included in the study. Seventy-nine of 390 patients (20.3%) had HCC and 311 did not have HCC. None of the 79 patients with HCC were lost to follow up. A total of 20 of 311 patients who did not have HCC were lost to follow up from the Veterans Affairs network, but were included in the analysis (Figure 1).
Univariate analysis
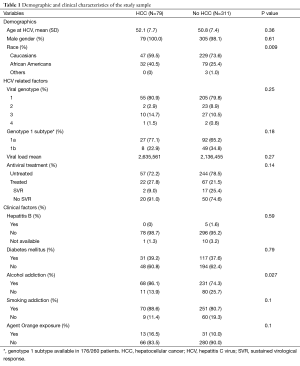
Table 1 shows the comparisons between those with HCC (N=79) and those without HCC (N=311). The HCC group did not differ from the non-HCC group on mean age (52.1±7.7 vs. 50.8±7.4, P=0.36) or male gender (100% vs. 98.1%, P=0.61). HCC patients were more likely to be African American than non-HCC patients (40.5% vs. 25.4%, P=0.009).
Full table
The prevalence of diabetes mellitus did not differ between the HCC (39.2%) and non-HCC group (37.6%) (P=0.79). HCV viral genotype information was available in 325 patients (68 in the HCC group and 257 in the non-HCC group). Viral genotype 1 was found in 80.9% of HCC patients and 79.8% of non-HCC patients; the remaining approximately 20% in each group were distributed among viral genotypes 2 through 4 (P=0.25). Information on viral subtype was available in 176 of 260 patients within genotype 1. Of the patients with genotype 1, 67.6% had subtype 1a and 32.4% had subtype 1b without subtype differences between the HCC and non-HCC groups (P=0.18). Information on viral load was available in 347 patients (66 in the HCC group and 281 in the non-HCC group); mean viral load between the two groups did not differ (P=0.27).
Eighty-five patients (21.8%) received antiviral treatment. In the HCC group 2 of 22 (9.1%) attained SVR vs. 13 of 63 (20.6%) in the non-HCC group (P=0.34). HCC patients were more likely to be addicted to alcohol than non-HCC patients (86.1% vs. 74.3%, P=0.027). A trend toward significance was seen in the HCC group for exposure to Agent Orange (16.5% vs. 10.0%, P=0.10) and smoking addiction (88.6% vs. 80.7%, P=0.10).
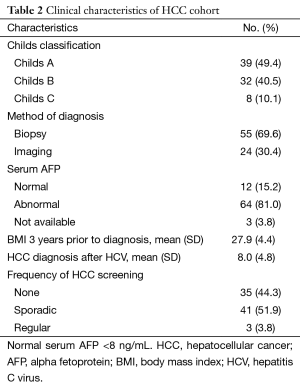
The clinical characteristics of the HCC cohort [n=79 (20.3%)] are shown in Table 2. Regarding the severity of their underlying liver disease, 49.4% were Childs A, 40.5% were Childs B, and 10.1% were Childs C. Biopsy was used to diagnose 69.6% of HCC patients while imaging was used less frequently (30.4%). Mean BMI 3 years prior to diagnosis of HCC was 27.9 (SD =4.4). HCC was diagnosed on average 8 (SD =4.8) years after the diagnosis of HCV in patients. Screening in the 3 years prior to diagnosis was evaluated, with only 3.8% (n=3) having regular screening at 6 monthly intervals. The majority had either sporadic screening [n=41 (51.9%)] or no screening [n=35 (44.3%)].
Full table
Multivariate analysis
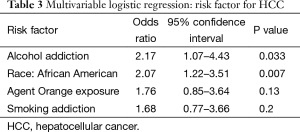
Using predictors that were significant or approaching significance (P≤0.10) from the univariate analysis, four factors were evaluated using a multivariate logistic regression model (Table 3): alcohol addiction, race (African American vs. Caucasian), Agent Orange exposure and smoking addiction.
Full table
The independent risk factors for HCC were African American race [odds ratio (OR) =2.07; 95% confidence interval (CI), 1.22–3.51; P=0.007] and alcohol addiction (OR =2.17; 95% CI, 1.07–4.43; P=0.033). Agent Orange exposure was not an independent risk factor (OR =1.76; 95% CI, 0.85–3.64; P=0.13). Tobacco abuse was not an independent predictor (OR =1.68; 95% CI, 0.77–3.66; P=0.20).
Discussion
HCC in the U.S. veteran population is affected by CHC and cirrhosis. Our study investigated multiple risk factors associated with HCC including exposure to Agent Orange. There have been several retrospective studies that evaluated risk factors for HCC in HCV cirrhotic patients in the U.S. veteran population (15,18,21-24). In our study, after a follow-up of 3,532 person-years, 20.3% developed HCC for an incidence rate of 22.37 per 1,000 person-years. On multivariate analysis, two factors, African American race and alcohol addiction, were found to be independent risk factors for the development of HCC in patients with HCV and cirrhosis. Our findings contrast with a prior study that showed the lowest incidence in African Americans and highest incidence in Hispanics (23). However, only 0.7% of our cohort with HCV and cirrhosis belonged to ethnicities other than Caucasians and African Americans, reflecting the composition of our local population.
Similar to our study, previous studies have found alcohol addiction to be independently associated with an increased risk of HCC (14,24). In our study smoking and HCC were not related.
In 180,251 South Korean Vietnam War veterans, Yi et al. examined the relationship of an Agent Orange exposure index, based on the geographic proximity of the veterans’ military unit to the area that was sprayed, to the risk for various cancers (20). A possible increased risk of liver cancer was found in this cohort. According to the U.S. Department of Veterans Affairs, about 2.5 million Americans served in Vietnam during the Vietnam War era, although it is not clear how many of them were exposed to Agent Orange. In our study, 11.3% of patients were exposed to Agent Orange without any significant association with HCC.
The seroprevalence of hepatitis B in our study cohort was 1.3%, similar to previous studies (21), but no difference in the prevalence of HCC was found between HCV mono-infection and Hepatitis B-HCV co-infection. While an association of HCC with a specific HCV genotype was not found in our study, one large study of U.S. veterans with both HCV and cirrhosis found HCC with genotype 3 to be related (15). A meta-analysis found those with HCV genotype 1b to have nearly double the risk of developing HCC as compared to other genotypes (17). In our study, 22.6% of patients diagnosed with HCV infection had been treated during the 14-year period of these 21.3% attained SVR, a rate similar to a large-scale study performed in U.S. veterans with HCV viremia (18). While some studies have showed an association of HCC with diabetes mellitus (12-14), others have not (24). We did not find a relationship between diabetes mellitus and HCC in our study cohort. The mean time from diagnosis of HCV to HCC was 8 years. Interestingly, the majority of patients with HCC had Childs A cirrhosis.
Our study was limited by its retrospective design and relatively small sample size from a single center. In addition, we excluded unconfirmed cases of HCV, which constituted 23% of HCV antibody positive patients with cirrhosis. The major strengths of our study were its long period of follow up and the evaluation of multiple variables associated with HCC. In addition, ours is the first study to examine the association between exposure to Agent Orange and HCC in U.S. veterans. We believe that our study included a representative sample of HCC patients with HCV and cirrhosis, as the prevalence of these conditions and the percentages treated are similar to prior studies (1,2).
Conclusions
The aim of our study was to evaluate multiple risk factors associated with HCC in patients with HCV and cirrhosis including exposure to Agent Orange. We found that African American race and alcohol addiction were independent risk factors for the development of HCC in a sample of U.S. veterans. In addition, our study is the first to examine the association between exposure to Agent Orange and HCC in U.S. veterans. There was no significant association between exposure to Agent Orange and HCC. However, larger studies are needed in the U.S. military veteran population to evaluate further this toxic herbicide from the Vietnam War era.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-73. [Crossref] [PubMed]
- Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001;34:809-16. [Crossref] [PubMed]
- Sangiovanni A, Del Ninno E, Fasani P, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 2004;126:1005-14. [Crossref] [PubMed]
- Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology 2005;41:88-96. [Crossref] [PubMed]
- Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep 2012;61:1-32. [PubMed]
- Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology 2011;140:1182-8. [Crossref] [PubMed]
- El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology 2002;36:S74-83. [Crossref] [PubMed]
- Walter SR, Thein HH, Gidding HF, et al. Risk factors for hepatocellular carcinoma in a cohort infected with hepatitis B or C. J Gastroenterol Hepatol 2011;26:1757-64. [Crossref] [PubMed]
- Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009;136:138-48. [Crossref] [PubMed]
- Degos F, Christidis C, Ganne-Carrie N, et al. Hepatitis C virus related cirrhosis: time to occurrence of hepatocellular carcinoma and death. Gut 2000;47:131-6. [Crossref] [PubMed]
- Ohki T, Tateishi R, Sato T, et al. Obesity is an independent risk factor for hepatocellular carcinoma development in chronic hepatitis C patients. Clin Gastroenterol Hepatol 2008;6:459-64. [Crossref] [PubMed]
- Veldt BJ, Chen W, Heathcote EJ, et al. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology 2008;47:1856-62. [Crossref] [PubMed]
- Davila JA, Morgan RO, Shaib Y, et al. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut 2005;54:533-9. [Crossref] [PubMed]
- El-Serag HB, Richardson PA, Everhart JE, et al. The role of diabetes in hepatocellular carcinoma: a case-control study among United States Veterans. Am J Gastroenterol 2001;96:2462-7. [Crossref] [PubMed]
- Kanwal F, Kramer JR, Ilyas J, et al. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology 2014;60:98-105. [Crossref] [PubMed]
- Lee MH, Yang HI, Lu SN, et al. Hepatitis C virus seromarkers and subsequent risk of hepatocellular carcinoma: long-term predictors from a community-based cohort study. J Clin Oncol 2010;28:4587-93. [Crossref] [PubMed]
- Raimondi S, Bruno S, Mondelli MU, et al. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol 2009;50:1142-54. [Crossref] [PubMed]
- McCombs J, Matsuda T, Tonnu-Mihara I, et al. The risk of long-term morbidity and mortality in patients with chronic hepatitis C: results from an analysis of data from a Department of Veterans Affairs Clinical Registry. JAMA Intern Med 2014;174:204-12. [Crossref] [PubMed]
- Van der Meer A, Feld J, Hofer H, et al. The risk for hepatocellular carcinoma among patients with chronic HCV infection and advanced hepatic fibrosis following sustained virological response: Abstract 143.64th annual meeting of the American Association for the Study of Liver Diseases, Washington, DC, 2013.
- Yi SW, Ohrr H. Agent Orange exposure and cancer incidence in Korean Vietnam veterans: a prospective cohort study. Cancer 2014;120:3699-706. [Crossref] [PubMed]
- Kruse RL, Kramer JR, Tyson GL, et al. Clinical outcomes of hepatitis B virus coinfection in a United States cohort of hepatitis C virus-infected patients. Hepatology 2014;60:1871-8. [Crossref] [PubMed]
- Ioannou GN, Bryson CL, Weiss NS, et al. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology 2013;57:249-57. [Crossref] [PubMed]
- El-Serag HB, Kramer J, Duan Z, et al. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. Am J Gastroenterol 2014;109:1427-35. [Crossref] [PubMed]
- Karagozian R, Baker E, Houranieh A, et al. Risk profile of hepatocellular carcinoma reveals dichotomy among US veterans. J Gastrointest Cancer 2013;44:318-24. [Crossref] [PubMed]