
Bedside ultrasound as an alternative to chest radiograph in detecting complications associated with central venous catheter placement: a retrospective cohort study
Introduction
Central venous catheter (CVC) placement is the most common procedure performed in the intensive care setting and is fundamental to the management of this patient population (1). In the USA, over 5 million of these procedures are performed each year (2). The most common complications associated with above diaphragm (i.e., subclavian or internal jugular vein) CVC placement are malposition and pneumothorax with rates previously reported to be up to 6.8% and 3.3%, respectively (3,4). Other complications including arterial puncture, hematoma, infection, and thromboembolic events are much less common (1). To minimize the most common adverse outcomes, obtaining a post-procedural chest radiograph (CXR) is routinely recommended (5).
After the introduction of ultrasonography (US) guidance in CVC placement, procedural success rate and safety have improved significantly (6,7). These improvements called into question the utility and advantages of obtaining a post-procedural radiograph after a clinically uncomplicated CVC insertion. Unlike bedside US, CXR has associated radiation exposure (8,9) and relatively low sensitivity (39.8%) for diagnosing such complications as pneumothorax (10). It is also a rather expensive modality (11) and is associated with delays in medical management. A meta-analysis by Ablordeppey et al. [2017] demonstrated that post-procedural US reduced the time of CVC confirmation by 58 min, as compared to post-procedural CXR (12). Lastly, obtaining a CXR requires specific personnel and equipment, which can be associated with an increased risk of infectious exposure and cross-contamination among patients and personnel.
Some of the studies aimed to assess the utility of post-CVC placement CXR have previously suggested that in the absence of clinical symptoms, CXR should not be considered as a reliable diagnostic method to assess for post-procedural complications (13). A recent large meta-analysis of the diagnostic accuracy of US to detect CVC malposition showed pooled specificity of 98.9% (95% CI: 97.8–99.5%) and sensitivity of 68.2% (95% CI: 54.4–79.4%) (13), making it a reasonable alternative to CXR. Chui et al. [2018], in a large population-based retrospective cohort study, concluded that the complication rates of CVCs placed with US guidance were very low (0.33% pneumothorax, 1.91% catheter misplacement) and the majority of these complications did not require any further interventions (11).
In light of this data, our study aims to compare rates of CVC malposition and iatrogenic pneumothorax following US-guided above diaphragm CVC placement using post-procedural bedside US versus using CXR ordered specifically for post-procedural purposes. This is a retrospective cohort study conducted exclusively in the intensive care unit (ICU) setting in one community hospital. To our knowledge, there is no study identical to our design. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jeccm-20-142).
Methods
The goal of this study is to determine whether the use of bedside US is inferior to post-procedural CXR (common standard of care) in detecting post-CVC placement complications including pneumothorax and line malposition. The second objective is to analyze the cost-effectiveness of obtaining chest radiographs specifically for post-procedural purposes. This is a retrospective cohort study conducted at a university affiliated community hospital. The hospital has twenty-four ICU beds, combined surgical, cardiovascular, and medical.
Participants
All participants were patients over 18 years of age and were admitted to the ICU at SSM St. Mary’s Hospital in St. Louis, MO and underwent CVC placement in the ICU setting. All CVCs placed in any setting other than ICU were excluded. Femoral vein sites and specialized catheters like dialysis catheters were excluded. All subjects underwent an US-guided CVC procedure using a 16 or 20 cm triple lumen catheter, secured in place per standard recommendations based on the site of insertion with sterile technique and appropriate safety measures in place. All ICU physicians involved in the study were trained in performing bedside US, certified in US-guided CVC placement technique, and had extensive knowledge and experience in these procedures.
Informed consent
We followed all the appropriate protocols through the SSM St. Mary’s Hospital Institutional Review Board (IRB) with their approval (18-12-1396). As this was a retrospective study that did not affect the current standard of practice in this intensive care unit and involved only data collection without any identifying information, it was considered ethical for the informed consent for participation to be waived. This decision was approved by the local IRB. Many CVC placement procedures were performed as an emergent measure so consents were presumed and documented but not obtained in written form. This study did not subject its participants to any increased risks as compared to non-participants and had no potential harm to either the patients or the staff members. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study groups
Before August 2018, all patients undergoing above diaphragm US-guided CVC insertion had a post-procedural CXR to evaluate for any immediate complications per facility protocol. In light of recent evidence, especially the study by Jason Chui, et al. [2018] (11) published in Chest, there was a shift towards less frequent ordering of post-procedural CXR. As a result, the intensivists decided that on August 17, 2018, the ICU will preferentially use post-procedural US as an alternative to post-procedural CXR. We therefore used August 17, 2018, as a separation point for the two study groups, before and after the intervention.
Intervention procedure
After August 17, 2018, all patients who underwent above diaphragm US-guided CVC placement were assessed by the physician performing the procedure to determine whether the procedure was complicated or uncomplicated. The basis of this assessment included but was not limited to the number of placement attempts, resistance during the needle placement or guidewire advancement, unexplained hemodynamic instability, excess bleeding, or arterial appearance of blood return. If the physician performing the procedure deemed it complicated, post-procedural CXR was obtained. In this study group, all patients underwent a pre- and post-procedural assessment of lung sliding to exclude the presence of pneumothorax, which is defined by presence of air separating the visceral and parietal pleura and blocking visualization of the visceral pleura (14). Some physicians also chose to obtain post-procedural CXR based on personal preference only, in the absence of any suspected complications or difficulties.
Data collection
The list of patients was created by a medical record liaison, who searched the database for all the CVCs placed from March 21, 2018 – January 21, 2019. This date range was chosen to account for 10 months with an approximately even dividing point on August 17, 2018. The search was conducted using ICD10 code for CVC placement. This list was stored in a Health Insurance Portability and Accountability Act (HIPPA) protected interface and shared with primary investigators with the only patient identifier being the medical record number and the date of the line placement. Upon the record review by the investigators, the data without any patient identifiers were recorded in a password protected spreadsheet.
In order to collect the data, the investigator opened the patient chart using the MRN and located the appropriate patient encounter based on the date. He/she then read through appropriate procedure notes and also reviewed the “imaging” section to collect the following data points: site of insertion, presence of pneumothorax, evidence of malposition, whether there was a CXR ordered specifically as a post-procedural order, whether CXR was obtained after the CVC placement for any other reason, the total number of CXRs performed for the patient during the hospital stay and the number of hospital days.
All CVC tips that were identified on imaging to be in any position other than superior vena cava (SVC) or cavoatrial junction were documented. These patient charts were further reviewed to assess whether this positioning required any adjustment, removal or was associated with any adverse outcomes. If the catheter position could not be assessed due to the absence of any imaging after the catheter placement, charts were analyzed for the presence of any issues that could be attributed to catheter malposition and for its subsequent removal or adjustment.
Analysis
Descriptive statistics were conducted for all variables with counts and percentages reported for categorical variables and means and standard deviations (SD) reported for continuous variables. Subjects were split into two groups, pre-intervention (before August 17, 2018) and post-intervention (on/after August 17, 2018). A chi-square examined differences between pre- and post-intervention for categorical variables, with Fisher’s exact test being reported when necessary for small cell size. An independent samples t-test was utilized for examining the differences between the continuous variables by the intervention period. Analysis was conducted in IBM SPSS v. 26 (IBM Corp. Released 2018. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.), at a significance level of 0.05.
Results
The original list of CVC procedures included 331 lines, 169 pre-intervention, and 162 post-intervention. In the post-intervention group, 132 records met the inclusion criteria. Based on this number, 132 records meeting the inclusion criteria were selected for further analysis from the pre-intervention group. The selection was based only on the date of the intervention and all patients were included starting from August 17, 2018, and working backward to obtain the total number of 132 records. The final date range was April 5, 2018 – January 21, 2019. After August 17, 2019, 77.3% of lines did not have a post-procedural CXR obtained, compared to 0.8% before that date.
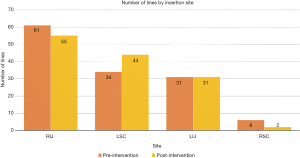
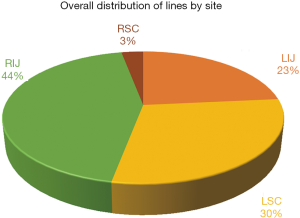
The distribution of lines by the site of placement is displayed in Figure 1 and 2. The most common site was right internal jugular vein (RIJ), followed by left subclavian vein (LSC) and left internal jugular vein (LIJ). There was an even distribution of lines by site of insertion between the pre- and post-intervention groups as shown in the figures.
The incidence of pneumothorax and line malposition identified on CXR is summarized in Table 1. There were two patients with pneumothorax with LIJ and LSC site of insertion in the pre-intervention group and one patient with pneumothorax with LIJ insertion site in the post-intervention group. None of these pneumothoraces were considered directly related to CVC placement. One patient had undergone cardiopulmonary resuscitation (CPR) and had rib fractures on the side of pneumothorax, one had several thoracentesis procedures and chest tube placement, and one had pneumothorax on the side opposite from the catheter insertion site.

Full table
Overall incidence of line malposition was 6.8% pre-intervention and 6.1% post-intervention, P=0.70. The most common site of insertion associated with malposition was LIJ, followed by RIJ and RSC/LSC, in both groups. Only one CVC in each group that was described as malpositioned was subsequently retracted. None of the catheters identified as malpositioned were found to be directly associated with any adverse outcomes and were used without restriction. There were 34 (12.9%) CVCs in the post-intervention group described as “unknown” positioning as these patients did not have any radiographic imaging after the CVC placement. None of the catheters in the pre-intervention group had an unknown positioning. The descriptions of line malposition are summarized in Table 2.

Full table
The length of hospital stay did not differ between the pre- and post-intervention groups (13.4, SD 10.1 vs. 13.1, SD 10.9, respectively), P=0.84. The mean number of CXRs per patient per hospital stay decreased from 4.7 (SD 3.3) pre-intervention to 3.5 (SD 2.8) post-intervention, P=0.002. The total number of CXRs (including post-procedural and all others) performed on the participants for the examined period was 614 pre-intervention and 459 post-intervention. Overall, 166 (62.9%) patients had a CXR obtained after the CVC placement but for a reason other than post-procedural assessment [82 (62.1%) pre-intervention, 84 (63.6%) post-intervention, P=0.9].
Cost analysis
The administrative database of this hospital described the cost of a portable CXR in the ICU to be approximately $244, while the post-procedural US is considered part of the CVC placement procedure and is free of additional charge. It requires the use of the same ultrasound machine and same provider who is performing the CVC placement procedure. Based on the total number of CXRs, the cost of these studies in the pre-intervention group was $149,816, compared to $111,996 post-intervention. This difference equates to the savings of approximately $37,820 over five months in this study population.
Discussion
This study showed no significant difference between the rates of complications associated with US-guided CVC placement in our ICU between the group that did have a routine post-procedural CXR and the one that did not. The overall rates of pneumothorax (1.1%) and line malposition (6.4%) were low. In the case of pneumothorax, all three cases could not be directly attributed to the CVC procedure and were recorded as non-iatrogenic. None of them required further intervention. There were no cases of traumatic subcutaneous emphysema or any other related complications. The high incidence of line malposition can be explained by the definition used. We counted all lines that were in any position other than the SVC or cavoatrial junction, which is a subjective assessment that was not used in other studies (4,15). Importantly, only two of the CVCs (one in each group) that were identified as malpositioned required retraction. None of the non-ideally positioned lines led to any complications or interfered with the ability to use the CVC.
As expected from the study design, 77.3% of patients in the post-intervention group did not have a radiograph obtained specifically post-procedure. Therefore, there was still a proportion of patients that had a CXR in addition to US, whether due to procedural difficulties or physician preference. Importantly, 62.9% of all patients had a CXR obtained after the CVC placement but for a reason other than post-procedural assessment, with no difference between the study groups. This means that majority of patients underwent a chest radiograph regardless of whether it was related to the CVC placement. It also means that physicians were not inclined to obtain more CXRs later on in the hospital course because the patient did not have a post-procedural one. Following previous studies, the most common placement site was RIJ (11). The most common site associated with malposition was found to be LIJ.
Overall, the mean number of CXRs per patient per hospital stay decreased significantly after the intervention. The total number of CXRs performed on patients after the change in practice was also lower. The cost analysis showed that post-CVC placement CXR is associated with substantial financial expenditure that does not justify its utility in our hospital setting. Considerable health care savings are possible if this and other studies are applied nationally.
One major issue identified in this study was a large proportion of patients in the post-intervention group in which the position of the line remained unknown due to lack of chest imaging following the CVC placement. This poses a risk of using a malpositioned CVC and can lead to patient-related complications. Reassuringly, none of the patients whose catheters were identified to be in an unknown position had any catheter-related complications based on chart review and these catheters were used without difficulties. There are additional ways to identify the position of a CVC. In a recent study, transthoracic echo with a bubble test was shown to be a feasible and accurate test to identify tip location in patients with atrial arrhythmia (16). Right atrial electrocardiography and electrocardiogram guidance have also been used (17,18). Further studies may be useful to compare these techniques with post-procedural US.
Another problem was that the data collection included a specific CPT code for the non-tunneled catheter placement (CPT 36556). As we discovered during the analysis, some lines failed to be documented under this code and therefore were not included. To keep pre- and post-intervention groups similar, we continued to analyze the lines only under this specific code, which was believed to minimize selection bias. We acknowledge that there may have been a small number of CVCs placed in the ICU that were not included in the study. Lastly, there was a potential for information bias, as patient charts were independently analyzed by the researchers retrospectively. To minimize this, we used standard aspects of the charts to be identified for later analysis. We also used physician and other personnel notes to identify any missed complications related to the procedures, in addition to the radiologist reading of the imaging. This strategy also helped to minimize the potential imbalance of prognostic factors between the two groups.
The recommendation to obtain a post-procedural CXR should include the physician’s suspicion for the catheter to be malpositioned. For example, if the procedure was difficult to perform or required multiple attempts, it is reasonable to obtain a post-procedural CXR to ensure correct positioning. There is less utility in obtaining the CXR to identify a pneumothorax if the immediate post-procedural bedside US has already ruled it out. This finding follows previous studies that have suggested that CXR is a suboptimal diagnostic tool for pneumothorax assessment (11,12,15).
This study also did not account for different patient characteristics that could potentially lead to higher rates of complications, such as body habitus. It is possible that CVC placement procedures performed on patients with higher body mass indexes are more difficult and may require a follow up CXR. There have been no studies to date that investigated this particular question and further research on this matter may be beneficial.
This study validated an evidence-based practice change in our facility. We believe that its findings can be applied to other community hospitals and extrapolated to larger institutions. Using US rather than CXR to validate US-guided CVC placement may be associated with reduced radiation exposure and substantial resource savings without posing any additional harm to the patient. In light of the recent coronavirus pandemic, a reduction in the number of radiographs would be valuable to conserve personal protective equipment and reduce patient exposure and interpersonal contact. Recently, there have been studies that explored the value of post-procedural CXR in other populations. The study by Cunningham et al. [2020], for example, recommended abandoning routine postoperative CXRs after image-guided CVC placement in asymptomatic children (19). Despite growing evidence against this practice, many institutions and physicians continue to use it. A recent survey study of emergency and critical care providers showed that the participants did not frequently use US for CVC confirmation. This was likely to be associated with the lack of appropriate US training, leading to less confidence in using this method (20).
Conclusions
This study supports the inclusion of post-procedure US in CVC placement procedural protocol. In addition, it shows that routine post-procedure CXR provides no benefit to the patient if post-procedure US is utilized. Current practice in most institutions continues to include a routine post-procedural CXR after CVC placement. Our findings demonstrated that catheter malposition and pneumothorax are rare after the US-guided CVC placement. We, therefore, in agreement with other similar studies, recommend against this practice. In our institution, we will continue to avoid post-procedural CXR use. This study challenges current practices and provides insight into the direct application of previous research findings into a large community-based setting. If the use of CXR can be reduced and/or eliminated in other institutions, it can lead to substantial reductions in cost, radiation exposure, and diagnostic time, leading to improvement in patient care. This study provides guidance for further development of standardized protocols for US-guided CVC placement, which could be cost-effective and safe for nationwide implementation.
Acknowledgments
Madan Chilappa, Amar Jadhav, Ankit Nahata.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jeccm-20-142
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jeccm-20-142
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jeccm-20-142). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). We followed all the appropriate protocols through the SSM St. Mary’s Hospital Institutional Review Board (IRB) with their approval (18-12-1396). As this was a retrospective study that did not affect the current standard of practice in this intensive care unit and involved only data collection without any identifying information, it was considered ethical that the informed consent for participation to be waived. This decision was approved by the local IRB. This study did not subject its participants to any increased risks as compared to non-participants and had no potential harm to either the patients or the staff members.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med 2003;348:1123-33. [Crossref] [PubMed]
- Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med 2007;35:1390-6. [Crossref] [PubMed]
- Nayeemuddin M, Pherwani AD, Asquith JR. Imaging and management of complications of central venous catheters. Clin Radiol 2013;68:529-44. [Crossref] [PubMed]
- Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med 2015;373:1220-9. [Crossref] [PubMed]
- Frykholm P, Pikwer A, Hammarskjöld F, et al. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiologica Scandinavica 2014;58:508-524. [Crossref] [PubMed]
- Adhikari S, Theodoro D, Raio C, et al. Central Venous Catheterization: Are We Using Ultrasound Guidance? J Ultrasound Med 2015;34:2065-70. [Crossref] [PubMed]
- Brass P, Hellmich M, Kolodziej L, et al. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev 2015;1:CD006962 [Crossref] [PubMed]
- Galante O, Slutsky T, Fuchs L, et al. Single-operator ultrasound-guided central venous catheter insertion verifies proper tip placement. Crit Care Med 2017;45:e994-e1000. [Crossref] [PubMed]
- McComb BL, Chung JH, Crabtree TD, et al. ACR Appropriateness Criteria Routine Chest Radiography. J Thorac Imaging 2016;31:W13-5. [Crossref] [PubMed]
- Alrajab S, Youssef AM, Akkus NI, et al. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care 2013;17:R208. [Crossref] [PubMed]
- Chui J, Saeed R, Jakobowski L, et al. Is Routine Chest X-Ray After Ultrasound-Guided Central Venous Catheter Insertion Choosing Wisely? A Population-Based Retrospective Study of 6,875 Patients. Chest 2018;154:148-56. [Crossref] [PubMed]
- Ablordeppey EA, Drewry AM, Beyeret AB, et al. Diagnostic Accuracy of Central Venous Catheter Confirmation by Bedside Ultrasound Versus Chest Radiography in Critically Ill Patients: A Systematic Review and Meta-Analysis. Crit Care Med 2017;45:715-24. [Crossref] [PubMed]
- Abood GJ, Davis KA, Esposito TJ, et al. Comparison of routine chest radiograph versus clinician judgment to determine adequate central line placement in critically ill patients. J Trauma 2007;63:50-6. [Crossref] [PubMed]
- Husain LF, Hagopian L, Wayman D, et al. Sonographic diagnosis of pneumothorax. J Emerg Trauma Shock 2012;5:76-81. [Crossref] [PubMed]
- Smit JM, Raadsen R, Blans MJ, et al. Bedside ultrasound to detect central venous catheter misplacement and associated iatrogenic complications: a systematic review and meta-analysis. Crit Care 2018;22:65. [Crossref] [PubMed]
- Iacobone E, Elisei D, Gattari D, et al. Transthoracic echocardiography as bedside technique to verify tip location of central venous catheters in patients with atrial arrhythmia. J Vasc Access 2020;21:861-7.
- McGee WT, Ackerman BL, Rouben LR, et al. Accurate placement of central venous catheters: a prospective, randomized, multicenter trial. Crit Care Med 1993;21:1118-23. [Crossref] [PubMed]
- Krishnan AK, Menon P, Gireesh Kumar KP, et al. Electrocardiogram-guided technique: an alternative method for confirming central venous catheter tip placement. J Emerg Trauma Shock 2018;11:276-81. [Crossref] [PubMed]
- Cunningham AJ, Haag MB, McClellan KV, et al. Routine Chest Radiographs in Children After Image-Guided Central Lines Offer Little Diagnostic Value. J Surg Res 2020;247:234-40. [Crossref] [PubMed]
- Tran QK, Foster M, Bowler J, et al. Emergency and critical care providers’ perception about the use of bedside ultrasound for confirmation of above-diaphragm central venous catheter placement. Heliyon 2020;6:e03113 [Crossref] [PubMed]
Cite this article as: Prishchepova A, Abramovitz AM, Dudaie RS, Buchanan PM. Bedside ultrasound as an alternative to chest radiograph in detecting complications associated with central venous catheter placement: a retrospective cohort study. J Emerg Crit Care Med 2021;5:11.


