18F-FDG PET/MRI in the diagnosis of an infected aortic aneurysm
Informed consent was obtained from the patient. A 64-year-old man with history of smoking, alcohol abuse and peripheral artery disease presented at the Emergency department with intense pain in the lower back after a 2-week history of fever, weight loss and drenching sweats.
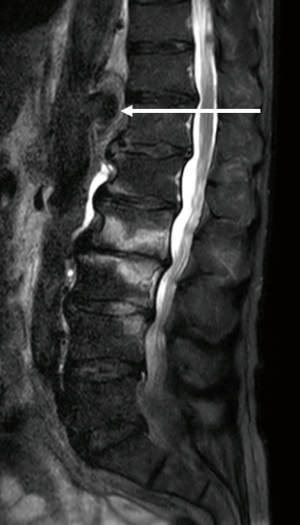
Systemic examination was otherwise unremarkable, there were no peripheral stigmata of infective endocarditis, auscultation of the precordium identified no murmurs, chest radiography and urinalysis were negative. Blood tests revealed elevated white blood cell count (11.1×109 cells/L) and C-reactive protein (103 mg/L). The patient was admitted to the ward and blood cultures were taken, which detected no bacteremia or fungemia. A magnetic resonance imaging (MRI) study of the patient’s back was performed to evaluate for possible spondylodiscitis. The study showed bone marrow edema and contrast enhancement of the lumbar vertebra L2, L3 and L4, indicative of active spondylodiscitis. As an additional finding, a saccular aneurysm protruding from the dorsal border of the abdominal aorta was observed (Figure 1).
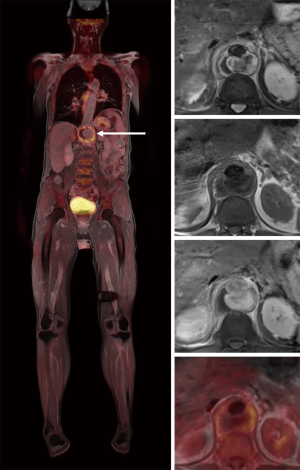
An integrated whole body 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/MRI (PET/MRI) study was performed to evaluate the extent of the suspicious aortic lesion, its relation to the spondylodiscitis as well as for other unknown foci of infection. The 18F-FDG PET/MRI study consisted of a whole body T2 weighted study, a whole body T1 weighted study pre- and post- contrast as well as of a whole-body PET study. All MR images were performed under breathing instructions. A T2 weighted study with fat suppression and T1 weighted study pre- and post-contrast in thin slices and with cardiac triggering were performed in the area of the aortic lesion. The study was performed on a Siemens Biograph mMR 3T scanner. A total dosage of 133 MBq (18F-FDG) and 10 mL MRI-contrast (Gadovist) was administered. PET/MRI images showed a saccular aortic aneurysm of 5.6 cm, extending from the distal thoracic aorta to the level of the celiac trunk and mesenteric artery origins (type V TAAA according to modified Crawford classification). Intense 18F-FDG uptake in the vessel wall of the aortic aneurysm reaching to a maximum standard uptake value (SUVmax) of 5.7 was seen as well as contrast enhancement within the thickened aortic wall and surrounding soft tissue (Figure 2).
The patient received intravenous antibiotics for ten days in total. The infected aortic tissue from the distal thoracic aorta to the level of the mesenteric artery origin was resected surgically by an open approach and the aneurysm was replaced by a tube graft made of bovine pericardium. The superior mesenteric artery and celiac trunk were directly re-implanted to the graft. Genotypic analysis of the aortic wall tissue surgical specimen by PCR revealed the presence of Gram-negative bacteria (Capnocytophaga canimorsus), possibly transmitted through a bite from the patient’s dog. The patient made a quick recovery. Post-surgery follow-up computed tomography imaging and blood tests revealed no signs of surgical complications and no signs of residual aortic or graft infection as well as a normalization of the CRP.
Integrated 18F-FDG PET/MRI has proven added value for the evaluation of different types of cancer (1-3). This case shows the high potential of FDG PET/MRI in the diagnostic algorithm of aortic lesions suspicious for infected aneurysms and other infectious spread. This diagnostic tool can have direct and significant impact on the therapeutic care pathway.
Mycotic aneurysms are a rare entity, but may pose a challenging problem (4). Survival is markedly increased by prompt diagnosis and surgical treatment (5,6). In early reports, most infected aneurysms were related to valvular infection, however nowadays most mycotic aneurysms are aortitis related (7,8). Capnocytophaga canimorsus is found in the oral flora of most dogs and is a rare pathogen in humans. Human infections with C. canimorsus have been previously reported, including endocarditis, abscesses, and mycotic aneurysms. Immuno-incompetent patients are at higher risk of infections (9). Other organisms with affinity to the aortic wall include a large variety of gram-positive and gram-negative species of which staphylococcus species (S. Aureus, S. pneumoniae, S. epidermidis) and salmonella species being most commonly detected. Less common pathogens include Coxiella burnetii (Q fever) and mycobacteria (10). Bacterial seeding of the aortic wall can occur by haematogenous spread, lymphatic spread or direct extension from an adjacent infected focus (8). The intimal lining of the aorta is generally highly resistant to infection, however, even normal aortic intima can be subject to bacterial invasion followed by secondary degeneration of the arterial wall with aneurysm enhancement and rupture. In one study, gram negative bacteria seemed to be associated with a higher risk of aortic rupture and mortality than gram-positive (11).
The diagnosis of mycotic aneurysm is difficult, particularly in the absence of the classical signs (back or abdominal pain, fever, pulsatile mass, positive blood culture). Yet, an early diagnosis is critical as mycotic infectious aortitis is associated with a high rate of rupture and subsequent mortality as early as one week after the onset of aortitis (12).
MR imaging with gadolinium enhancement is becoming the non-invasive imaging modality of choice for aortitis (13). MR imaging demonstrates the anatomic localization, degree and extent of vascular stenosis or vascular aneurysmal dilatation. Moreover, it has an excellent resolution of the aortic wall and it can depict areas of active aortitis as crescentic or ring-like vessel wall oedema and/or thickening (14). Drawbacks of MRI are the relatively decreased sensitivity as well as a long procedure time.
More recently, the use of 18F-FDG has emerged as a potential tool for the initial diagnosis and assessment of disease activity of aortitis caused by either infectious conditions (15,16) or non-infectious giant cell arteritis or Takayasu arteritis (3,17-20). After first positive results of 18F-FDG PET imaging in infectious/mycotic aortic aneurysm (15,16), recent imaging series have reported a variable sensitivity of 60–90% and a specificity of 88–100% of 18F-FDG PET and PET CT for diagnosing active inflammation in arteritis (17,20-22). Anatomic changes, such as the presence of wall thickening, luminal thrombus and aneurysm size cannot be assessed on PET. Hence, by combining the advantages of both MRI and PET, the hybrid PET/MRI imaging is thought to be an imaging modality that could improve the sensitivity in the diagnosis of aortitis.
In conclusion, the integrated whole body 18F-FDG PET/MRI study provided all relevant diagnostic imaging information needed for the diagnosis of an infected aortic aneurysm and the successful planning of its treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Informed consent was obtained from the patient.
References
- Kang B, Lee JM, Song YS, et al. Added Value of Integrated Whole-Body PET/MRI for Evaluation of Colorectal Cancer: Comparison With Contrast-Enhanced MDCT. AJR Am J Roentgenol 2016;206:W10-20. [Crossref] [PubMed]
- Lee DH, Kim SH, Im SA, et al. Multiparametric fully-integrated 18-FDG PET/MRI of advanced gastric cancer for prediction of chemotherapy response: a preliminary study. Eur Radiol 2016;26:2771-8. [Crossref] [PubMed]
- Grueneisen J, Schaarschmidt BM, Heubner M, et al. Integrated PET/MRI for whole-body staging of patients with primary cervical cancer: preliminary results. Eur J Nucl Med Mol Imaging 2015;42:1814-24. [Crossref] [PubMed]
- Gornik HL, Creager MA. Aortitis. Circulation 2008;117:3039-51. [Crossref] [PubMed]
- Foote EA, Postier RG, Greenfield RA, et al. Infectious Aortitis. Curr Treat Options Cardiovasc Med 2005;7:89-97. [Crossref] [PubMed]
- van der Vaart MG, Meerwaldt R, Slart RH, et al. Application of PET/SPECT imaging in vascular disease. Eur J Vasc Endovasc Surg 2008;35:507-13. [Crossref] [PubMed]
- Brown SL, Busuttil RW, Baker JD, et al. Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J Vasc Surg 1984;1:541-7. [Crossref] [PubMed]
- Ishizaka N, Sohmiya K, Miyamura M, et al. Infected aortic aneurysm and inflammatory aortic aneurysm--in search of an optimal differential diagnosis. J Cardiol 2012;59:123-31. [Crossref] [PubMed]
- Evans TJ, Lyons OT, Brown A, et al. Mycotic Aneurysm following a Dog Bite: The Value of the Clinical History and Molecular Diagnostics. Ann Vasc Surg 2016;32:130.e5-7. [Crossref] [PubMed]
- Moneta GL, Taylor LM Jr, Yeager RA, et al. Surgical treatment of infected aortic aneurysm. Am J Surg 1998;175:396-9. [Crossref] [PubMed]
- Jarrett F, Darling RC, Mundth ED, et al. Experience with infected aneurysms of the abdominal aorta. Arch Surg 1975;110:1281-6. [Crossref] [PubMed]
- Reddy DJ, Shepard AD, Evans JR, et al. Management of infected aortoiliac aneurysms. Arch Surg 1991;126:873-8; discussion 878-9. [Crossref] [PubMed]
- Kissin EY, Merkel PA. Diagnostic imaging in Takayasu arteritis. Curr Opin Rheumatol 2004;16:31-7. [Crossref] [PubMed]
- Tso E, Flamm SD, White RD, et al. Takayasu arteritis: utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum 2002;46:1634-42. [Crossref] [PubMed]
- Davison JM, Montilla-Soler JL, Broussard E, et al. F-18 FDG PET-CT imaging of a mycotic aneurysm. Clin Nucl Med 2005;30:483-7. [Crossref] [PubMed]
- Takahashi M, Momose T, Kameyama M, et al. Abnormal accumulation of [18F]fluorodeoxyglucose in the aortic wall related to inflammatory changes: three case reports. Ann Nucl Med 2006;20:361-4. [Crossref] [PubMed]
- Kobayashi Y, Ishii K, Oda K, et al. Aortic wall inflammation due to Takayasu arteritis imaged with 18F-FDG PET coregistered with enhanced CT. J Nucl Med 2005;46:917-22. [PubMed]
- Meller J, Grabbe E, Becker W, et al. Value of F-18 FDG hybrid camera PET and MRI in early takayasu aortitis. Eur Radiol 2003;13:400-5. [PubMed]
- Meller J, Strutz F, Siefker U, et al. Early diagnosis and follow-up of aortitis with [(18)F]FDG PET and MRI. Eur J Nucl Med Mol Imaging 2003;30:730-6. [Crossref] [PubMed]
- Webb M, Chambers A. The role of 18F-FDG PET in characterising disease activity in Takayasu arteritis. Eur J Nucl Med Mol Imaging 2004;31:627-34. [Crossref] [PubMed]
- Walter MA, Melzer RA, Schindler C, et al. The value of [18F]FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging 2005;32:674-81. [Crossref] [PubMed]
- Soussan M, Nicolas P, Schramm C, et al. Management of large-vessel vasculitis with FDG-PET: a systematic literature review and meta-analysis. Medicine (Baltimore) 2015;94:e622. [Crossref] [PubMed]


