Complications of inferior vena cava filters
Introduction
The incidence of venous thromboembolism (VTE) has remained stable at 0.1% of the United States population experiencing their first deep vein thrombosis (DVT) or pulmonary embolism (PE) each year (1). This amounts to 300,000 people experiencing a PE each year in the United States. With a 12% likelihood of death within one month after development of a VTE, this represents a major source of morbidity and mortality. The first line treatment is anticoagulation, however, for many patients anticoagulation may be contraindicated or ineffective. For these patients, inferior vena cava (IVC) filter placement may become the treatment of choice. Currently, the Society of Interventional Radiology (SIR) and the American College of Chest Physicians (ACCP) have conflicting recommendations particularly in reference to the use of prophylactic filter placement in patients who have no history of VTE. The ACCP recommends placement only in patients with an acute DVT who cannot tolerate anticoagulation due to active bleeding or high risk of bleeding. The SIR states filters are indicated in patients who having a VTE and have some contraindication to anticoagulation or failure of anticoagulation. Additionally, SIR states prophylactic IVC filters in patients without current thromboembolic disease are indicated in patients with severe trauma or those at high-risk such as immobilized patients in the ICU setting. Ultimately, there has been an exponential increase in the use of IVC filters over the past two decades. An estimated 2,000 IVC filters were placed in 1979, with a 25-fold increase to nearly 50,000 filters in 1999 (2). Recently, placement rates range from 12% to 17% of all patients with VTE in USA, which is notably 25 times higher than the usage rates in Europe (3). A significant portion of this rise came after the early 2000s when the United States Federal Drug Administration (FDA) approved the use of retrievable IVC filters. As of 2012, it was estimated that 75% of all newly placed filters were retrievable devices, and more than half were placed for prophylactic indications (4). However, with this rising utilization of IVC filters, questions of safety and indications for use have been raised. In light of the exponential rise in their use, the specific aims of this review are to outline the complications associated with IVC filters as well as the specific incidences, symptomatology, prevention and treatment strategies. Differences amongst device types, retrievable and permanent filters are also addressed. The overall complications of IVC filters are divided into procedure related complications, post-procedure complications and complications associated with retrieval of the filter device.
Procedure complications
Vascular access complications
Complications from vascular access for IVC filter insertion have been reported at a rate of 4–11% (5). The complications and incidence rates are similar to those with central venous catheter insertion. The most common complications of vascular access are bleeding and access site thrombosis. Bleeding at the access site is seen in 6–15% of patients (6). Though common, significant bleeding requiring transfusion or surgical intervention is very rare in published literature suggesting it carries a low morbidity. Incidence of thrombosis at the access site ranges between 2–35% and occurs more commonly in patients with a known underlying hypercoagulability (7). Variability stems from whether follow-up evaluations included ultrasound imaging. Molgaard et al. demonstrated a 35% incidence when ultrasound at 1-month follow-up was performed of which only 3% were symptomatic (8). Clinical relevance of asymptomatic cases is unknown as vein patency is preserved in the vast majority. Treatment should include anticoagulation, if possible, with the expectation that most will resolve on their own. For prevention, vessel manipulations should be minimized in the peri-procedural period. Though pressure over the insertion site after the removal of the sheath limits bleeding, this should not be prolonged. No studies have directly compared variability in bleeding or access site thrombosis across device types to date though incidence rates appear similar across separate studies (9-11).
Arteriovenous fistula (AVF) is a rare complication of IVC filters thought to arise from trauma to adjacent arteries during the procedure. The reported incidence rate based upon review of published case series up to 2004 was 0.02% (12). Diagnostic imaging is needed to confirm the presence of an AVF and this may have led to an underreporting of the incidence due to asymptomatic cases. Patient may present with localized pain, vasodilatation, ischemia distal to lesion, and a palpable thrill. Early treatment is preferred due to the risk of enlargement over time. Embolization or surgical management can be performed. No device specific differences have been published.
Filter complications
Filter tilt
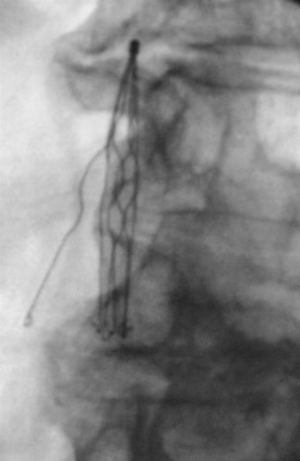
Presence of IVC filter tilt is defined as greater than 15 degrees’ angulation of the filter from the long axis of the vena cava (Figure 1). This can be seen with all filters except for the Bird’s Nest Filter (Cook Medical, Bloomington, IN, USA) secondary to the design. Tilt was found to be the most common cited cause for failure to retrieve filters (5). Rogers et al. found that filters tilted greater than 14 degrees had an association with an increase in PE and recommended addition of a second filter for adequate prophylaxis (7,13). Studies on Greenfield IVC filters (Boston Scientific, Natick, MA, USA) showed an incidence of tilt in 5% of filter placements and a trend toward increased PE and IVC thrombosis in tilted versus non-tilted filters. The difference was not statistically significant, however, it was likely limited by small study size (5,14). The increase in tilting during deployment of Greenfield filters occurred with the original design and over-the-wire Greenfield filter was designed to address this complication (15). In a review of retrievable IVC filters, tilting was most commonly associated with the Recovery/G2 (Bard Peripheral Vascular, Tempe, AZ, USA) and Günther-Tulip (Fort Wayne Metals, Fort Wayne, IN, USA) filters (16). In a comparison of retrievable versus permanent filters, the only case of filter tilt was found in a retrievable OptEase filter (Cordis Endovascular, Miami Lakes, FL, USA) (17). This was noted at the time of retrieval as the filter could not be removed due to the tilt. Of note, there was no screening of filter angle in the permanent filters. There is no increased risk of thrombosis with filter tilt of <15 degrees. For a tilt greater than or equal to 15 degrees, the treatment is controversial. Retrieval of the filter can be complicated as filter tilt may lead to embedment or perforation of the filter into the caval wall. Some advocate attempting to adjust position at the time of placement, however this is not recommended or approved by the manufacturers. Placement of a second filter is advocated by few but is not supported in the literature. A study performed on Celect filters (Cook, Bloomington, IN, USA) was able to employ a stiff guide wire to adjust the axis prior to deployment with success (18). Additionally, others choose expectant management as the filter positioning may improve spontaneously over time (14).
Filter migration
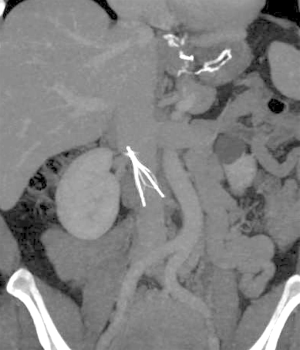
Significant filter migration is defined as a 2 cm or greater superior or inferior movement from the initial placement location. Filter migration can be due to a variety of causes. A filter being undersized for the vena cava may lead to migration. Most filters on the market are currently approved for vena cava of 28 mm or less in diameter with the exception of the Bird’s Nest filter which can be placed in a vena cava up to 40mm in diameter (7,9). Additionally, placement of central lines can dislodge a filter. This can be prevented by using fluoroscopic techniques when placing a central line in a patient with an IVC filter. The original Greenfield filter was associated with significant migration which prompted a design change in 1991 in which filter hooks were modified to prevent this complication. Before the discontinuation of the Mobin-Uddin filter in 1986, it was associated with high rates of migration which resulted in several deaths (12). With contemporary filters, migration rates are much lower with all filters having <1% incidence of migration except the G2 filter which has a 4.5% incidence of migration. It was found that 90% of migration cases were found greater than 30 days after initial placement (16). The Bird’s nest filter has not had any MRI documented migration, however, it was noted that the significant artifact caused by the filter limits visualization (9). In a comparison of retrievable versus permanent filters, migration was found two times more with permanent filters (19). Migration of the filter into the cardiopulmonary system (Figure 2) requires immediate intervention as this can have fatal consequences. An endovascular approach is preferred however open surgery may be required in few cases.
Incomplete opening of the filter
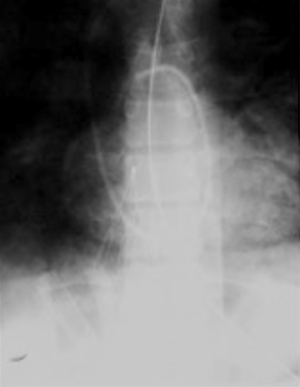
Incomplete opening of the filter can be due to a defect in the filter, operator error, or an unidentified thrombus in the IVC leading to an abnormal and asymmetric configuration of the filter following deployment as demonstrated in Figure 3. Incidence rates range from 0.7% to 13.9% (6). The reported incidence of incomplete opening of titanium Greenfield filters has been as high as 71% (20). Incomplete opening can lead to wide gaps in the filter which can lead to a reported 80% decrease in filtering efficacy particularly with thrombi less than 5 mm (21). Though some advocate manipulation of the filter legs, this is not recommended by the device manufacturers. Some advocate immediate removal after deployment as the adverse effects of an incompletely opened filter is not known (22).
Operator errors
Filter placement in non-target location
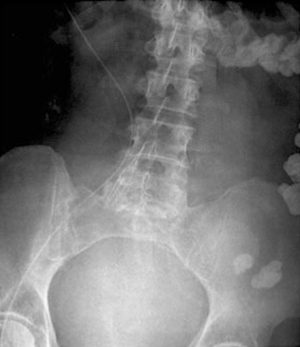
The preferred location for placement of an IVC filter is usually inferior to the lowest renal venous inflow and superior to the confluence of the common lilac veins. There is a preference to place the apex of the filter at the renal venous inflow to prevent superior extension of a thrombus captured by the filter. Placement of the filter in the suprarenal IVC may increase the likelihood of renal vein thrombosis especially in the presence of acute thrombosis of infrarenal IVC (23). Suprarenal placement may be used as prophylaxis against PE in the presence of renal or gonadal vein thrombosis or in a pregnant woman (24). Placement inferior to the confluence of the iliac veins leaves the contralateral iliac system unfiltered (Figure 4). Misplacement of an IVC filter was more common with surgical placement when directly compared to percutaneous placement (25). Beyond being misplaced within the vena cava, there have been reports of filter placement in an incorrect vessel. There have been case reports of filter placements in the gonadal veins, one such case leading to hydronephrosis and thrombosis of the ipsilateral ovarian vein (26). There have been reports of misplacements of filters in the mesenteric veins, in the aorta which was discovered on transesophageal echocardiogram, and even in the spinal column after perforation of the sheath into the retroperitoneum, vertebral foramina and ultimately the spinal canal (7,27,28). As such, cavagram prior to the insertion is vital to assess the vascular anatomy and correct placement of the filter. Additionally, selective venography may allow for further identification of aberrant anatomy and minimization of misplacement errors (29).
Incorrect orientation of the filter
In addition to incorrect location, the orientation of the filter is another potential area for complications to arise. Though rare, incorrect orientation of a filter has been reported. In one such case, an OptEase filter was placed incorrectly in an inverted orientation and required a combined jugular and femoral approach for retrieval (30). Another case of an inverted filter was associated with migration of an ALN filter (ALN implants Chirurgicaux, Ghisonaccia, France) to below the right atrium (31). As placement of a filter in the wrong orientation can cause the filter to both non-functional as well as difficult to retrieve. An upside down filter can be missed particularly with symmetric filter designs such as the OptEase if care is not taken to ensure proper orientation.
Post-procedure complications
Thrombosis
Caval thrombosis (Figure 5) has been cited to have a less than 10% incidence rate with contemporary filters but published rates range widely from 2–30% (7,32). The variability likely stems from screening practices of asymptomatic patients as this is ten times more common than symptomatic caval thrombosis (33). The symptoms include pain and edema of both lower extremities as well as renal failure if the thrombus extends in to the suprarenal IVC. A thrombosed IVC filter (Figure 6), may increase the risk of PE as thrombus may extend above the filter and then emboli leading to recurrent PE in the setting of an IVC filter (32). The exact etiology of filter thrombosis is unknown—it may be related to patients’ increased baseline risk for thromboembolism or to the filter’s inherent thrombogenicity as a foreign body in the cava. Additionally, a thrombus within the filter can represent a captured thrombi rather than an in situ thrombus formation. Comparisons across filter designs show overall similar rates of IVC thrombosis below 10% except for the TrapEase filter which has a reported incidence of 25% (6,9,11). However, this represents pooled retrospective data as no prospective randomized studies have been done. Retrievable filters have been shown to have a higher incidence when compared to permanent filters (19). No significant difference exists in patients with or without malignancy (34). Standard practice currently includes starting anticoagulation once caval thrombosis is discovered (35). When comparing those who received anticoagulation on detecting of IVC thrombus with those who did not, no significant difference in rates of progression or regression of the thrombus was found. Additionally, there was rarely progression to complete occlusion. Thus expectant management can be utilized for those whom anticoagulation presents a significant risk (33). Importantly, anticoagulation does not improve the rate of IVC thrombosis and should not be used prophylactically for this reason (12). Additionally, thrombosis of the filter can be managed by placing a stent across the filter opening up flow through the vena cava (36).
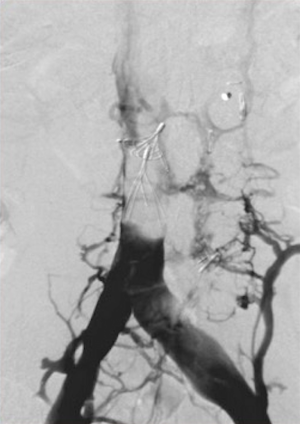
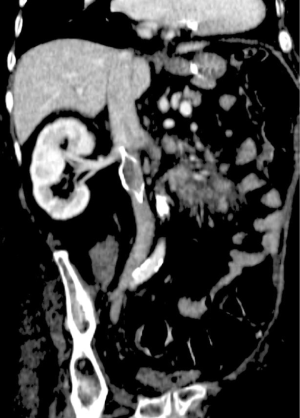
Deep venous thrombosis is a major delayed complication of IVC filters. Decousus et al. performed a randomized prospective study which found that filter recipients treated with anticoagulation were more likely to develop a DVT than patients treated with anticoagulation alone (37). Two years after filter placement there was a two-fold increased risk of DVT (12). The etiology for this increased risk has been postulated to be a combination of filter-induced changes in venous blood flow and stasis as well as the patient’s underlying coagulopathy. The incidence of DVT in filter recipients widely varies and is cited to be as high as 43% (7). Patients may be asymptomatic or have associated edema and skin changes. Retrievable filters were thought to be a solution to this complication, however, due to only a minority of filters being removed, the incidence of DVT is comparable to that of permanent filters (17,19). Across device types, VenaTech (B. Braun Medical Inc., Evanston, IL, USA), TrapEase, and ALN have the highest incidence rates and the Gunther Tulip the lowest (9,12). Ultimately IVC filter placement becomes a balance between the prevention of PE and an increased risk of VTE providing reason for prompt removal of retrievable filters as soon as indicated.
Filter fracture
Filter Fracture (Figure 7) occurs when there is a structural failure of a filter leading to fragmentation and potential embolization of the fragment. This is often a late complication of filters, most often seen after a filter has been in place for greater than one year (10). This relation with length of time implanted hints that this may be due to a structural fatigue of the filter over time. The overall incidence is 1–2% and is most commonly reported in the G2 filter based on the U.S. Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database (4,9). Filter fracture is the most common reported complication in the MAUDE database associated with retrievable filters and is significantly less common in permanent filters (4). There have been reports of migration of fractured fragments to the pulmonary vasculature, renal veins and heart (38-40). Figure 8 demonstrates a fracture associated with filter tilt. In these cases, the patients were asymptomatic. Upon discovery, removal of the filter and fragment is recommended. As this complication is more common with retrievable filters after long periods of dwell time, prompt removal of filters can be preventative of filter fracture. Currently, retrievable filters have a very low retrieval rate as filters are left in place well after they are indicated (4).
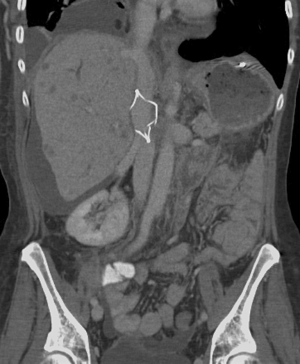
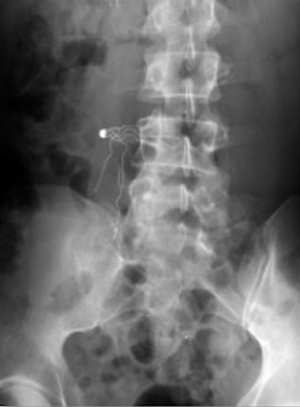
Perforation
Filter perforation is defined as when a filter component penetrates >3 mm of the wall of the vena cava and enters the peri-caval space and/or the adjacent structures. Perforation may occur immediately on deployment of a filter or as a late consequence. Movement of the IVC with aortic pulsations and respiration are thought to be the possible mechanisms for the latter delayed presentation. As hooks have been added to filters to decrease migration, perforation has consequently increased in incidence (9). Overall, 20% of the complications in the MAUDE database are accounted for by IVC perforations (16). Incidence of perforation varies widely with filter type and design. Greenfield, Bird’s Nest and Simon Ninitol (C.R. Bard, Covington, GA, USA) filters usually having the highest incidence of perforation (6,23). A study performed of Celect filters found a progressive increase in perforation rates with a 43% increase from initial to final CT scan (41). Retrievable filters have been found to have higher rates of perforation than the permanent filters, particularly when the retrievable filters are left in place longer than anticipated (19). Patients are usually asymptomatic. However, when adjacent structures are perforated by the filter, potentially severe clinical consequences may occur. Reports of perforation into the duodenum, the aorta (Figure 9), and renal pelvis have been reported (42-44). Concomitant anticoagulation in the setting of IVC filter perforation increases the risk of bleeding and may lead to retroperitoneal hematoma formation (45). A high clinical suspicion for perforation should be maintained with non-specific abdominal or back pain in patients with an IVC filter. Endovascular or open surgical retrieval can be performed depending on patient and physician preference. Expectant management for asymptomatic patients with close follow-up for complications or worsening perforation may be appropriate.
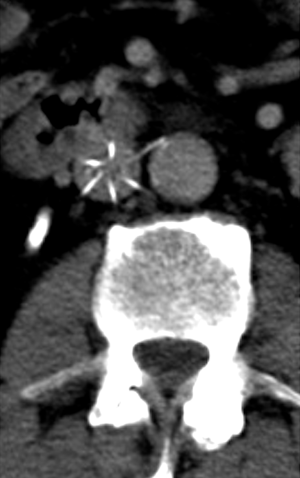
There have been a handful of case reports of lumbar artery pseudoaneurysms caused by IVC filters (46). Three have been with Greenfield filters and one with a G2 filter. The patients presented with lower back pain shortly after the procedure. These are treated preferentially with embolization. There has been a case report of an infrarenal aortic pseudoaneurysm after placement of a Simon Nitinol IVC filter (47). Additionally, an abdominal aortic pseudoaneurysm was reported from erosion of a Bird’s Nest IVC filter into the wall of the abdominal aorta (48). Both of these were resolved with surgical graft placement. Overall, the incidence of pseudoaneurysms as a complication from IVC filter placement represents an exceedingly rare complication with the potential for severe morbidity and mortality.
Filter retrieval complications
The advent of retrievable filters resulted in a new subset of IVC filter complications. Complications associated with IVC filter retrieval include filter fracture and IVC injury such as intussusception, dissection or hemorrhage. The MAUDE database contains 111 reports of complications that occurred during removal of retrievable filters (16). Studies consistently show increased rate of complication with retrievable filters when compared to permanent filters. Retrieval rates vary based on filter type, ranging widely from 100 percent to less than half Reasons for failure to remove included embedment, tilting and thrombosis of filter (4,16). Longer dwell times, increased tilt angles and hook embedment are significantly associated with increased complications associated with retrieval (49). Notably, only one of the reported complications in the MAUDE database occurred within 30 days of placement (16). Though prompt removal of filters is the most modifiable risk factor, longer dwell times is not a contraindication to retrieval of a filter. Numerous cases have shown retrieval of adherent and chronically implanted filters without any complications such IVC injury or fracture even when aggressive force is employed (50). A Gunther Tulip filter was removed 3,006 days after placement, the longest published dwell time prior to retrieval, without complication (51). Overall, prompt removal of filters as soon as indicated is recommended as the risks associated with filter retrieval are still favorable when compared to the risks of leaving a retrievable filter in place indefinitely.
Conclusions
With advancements in filter design, the incidence of complications associated with IVC filters has decreased. Of note, many of the complications listed above have asymptomatic presentation and therefore the true complication rates are likely under-reported. The clinical significance of these asymptomatic cases is however unknown. Although IVC filters have become an increasingly accepted as a low morbidity method of preventing PE there is little prospective data to guide physicians on which patients would benefit most. Additionally, there are sparse randomized control studies comparing the incidence of complications by device type. The Society of Interventional Radiology and the Society of Vascular Surgery are in the process of the PRESERVE (Predicting Safety and Effectiveness of Inferior Vena Cava Filters) study which will glean a prospective, multicenter, clinical trial aimed at addressing the safety and effectiveness of IVC filers. The advent of retrievable filters has brought along a new subset of filters with unique advantages as well as complication rates and considerations for the physician. In 2010, the FDA issued a safety alert due to the growing use of retrievable filters and the concomitantly increasing complication rate. This increase in complication rate with retrievable filters is likely linked to poor follow-up for removal and should be the focus of future quality improvement protocols. For the well selected patient with appropriate follow-up, IVC filter placement prevents potentially fatal PE events with a very low risk of complications. Ultimately, future prospective studies are necessary to enable physicians to practice prudent evidence based decisions about patient and therapy selection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1. Moore PS, Andrews JS, Craven TE, et al. Trends in vena caval interruption. J Vasc Surg 2010;52:118-125.e3; discussion 125-6.
- Stein PD, Matta F, Hull RD. Increasing use of vena cava filters for prevention of pulmonary embolism. Am J Med 2011;124:655-61. [Crossref] [PubMed]
- Alkhouli M, Bashir R. Inferior vena cava filters in the United States: less is more. Int J Cardiol 2014;177:742-3. [Crossref] [PubMed]
- Andreoli JM, Lewandowski RJ, Vogelzang RL, et al. Comparison of complication rates associated with permanent and retrievable inferior vena cava filters: a review of the MAUDE database. J Vasc Interv Radiol 2014;25:1181-5. [Crossref] [PubMed]
- Kinney TB. Update on inferior vena cava filters. J Vasc Interv Radiol 2003;14:425-40. [Crossref] [PubMed]
- Joels CS, Sing RF, Heniford BT. Complications of inferior vena cava filters. Am Surg 2003;69:654-9. [PubMed]
- Martin MJ, Blair KS, Curry TK, et al. Vena cava filters: current concepts and controversies for the surgeon. Curr Probl Surg 2010;47:524-618. [Crossref] [PubMed]
- Molgaard CP, Yucel EK, Geller SC, et al. Access-site thrombosis after placement of inferior vena cava filters with 12-14-F delivery sheaths. Radiology 1992;185:257-61. [Crossref] [PubMed]
- Carman TL, Alahmad A. Update on vena cava filters. Curr Treat Options Cardiovasc Med 2008;10:101-11. [Crossref] [PubMed]
- Kalva SP, Wicky S, Waltman AC, et al. TrapEase vena cava filter: experience in 751 patients. J Endovasc Ther 2006;13:365-72. [Crossref] [PubMed]
- Usoh F, Hingorani A, Ascher E, et al. Prospective randomized study comparing the clinical outcomes between inferior vena cava Greenfield and TrapEase filters. J Vasc Surg 2010;52:394-9. [Crossref] [PubMed]
- Hann CL, Streiff MB. The role of vena caval filters in the management of venous thromboembolism. Blood Rev 2005;19:179-202. [Crossref] [PubMed]
- Rogers FB, Strindberg G, Shackford SR, et al. Five-year follow-up of prophylactic vena cava filters in high-risk trauma patients. Arch Surg 1998;133:406-11; discussion 412. [Crossref] [PubMed]
- Greenfield LJ, Proctor MC, Cho KJ, et al. Limb asymmetry in titanium Greenfield filters: clinically significant? J Vasc Surg 1997;26:770-5. [Crossref] [PubMed]
- Kinney TB, Rose SC, Weingarten KE, et al. IVC filter tilt and asymmetry: comparison of the over-the-wire stainless-steel and titanium Greenfield IVC filters. J Vasc Interv Radiol 1997;8:1029-37. [Crossref] [PubMed]
- Angel LF, Tapson V, Galgon RE, et al. Systematic review of the use of retrievable inferior vena cava filters. J Vasc Interv Radiol 2011;22:1522-1530.e3. [Crossref] [PubMed]
- Kim HS, Young MJ, Narayan AK, et al. A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters. J Vasc Interv Radiol 2008;19:393-9. [Crossref] [PubMed]
- Knott EM, Beacham B, Fry WR. New technique to prevent tilt during inferior vena cava filter placement. J Vasc Surg 2012;55:869-71. [Crossref] [PubMed]
- Desai TR, Morcos OC, Lind BB, et al. Complications of indwelling retrievable versus permanent inferior vena cava filters. J Vasc Surg Venous Lymphat Disord 2014;2:166-73. [Crossref] [PubMed]
- Sweeney TJ, Van Aman ME. Deployment problems with the titanium Greenfield filter. J Vasc Interv Radiol 1993;4:691-4. [Crossref] [PubMed]
- Korbin CD, Van Allan RJ, Andrews RT, et al. Strut interlocking of titanium Greenfield vena cava filters and its effect on clot capturing: an in vitro study. Cardiovasc Intervent Radiol 1994;17:204-6. [Crossref] [PubMed]
- Kohi MP, Taylor AG, Kolli KP, et al. Crossed legs: an unexpected occurrence during an ALN filter placement. Clin Imaging 2015;39:1128-9. [Crossref] [PubMed]
- Ray CE Jr, Kaufman JA. Complications of inferior vena cava filters. Abdom Imaging 1996;21:368-74. [Crossref] [PubMed]
- Carrafiello G, Mangini M, Fontana F, et al. Suprarenal inferior vena cava filter implantation. Radiol Med 2012;117:1190-8. [Crossref] [PubMed]
- Hye RJ, Mitchell AT, Dory CE, et al. Analysis of the transition to percutaneous placement of Greenfield filters. Arch Surg 1990;125:1550-3. [Crossref] [PubMed]
- Tan WP, Sherer BA, Khare N, et al. Unfriendly Filter: An Unusual Cause of Hydronephrosis and Hematuria. Urology 2016;87:e9-e10. [Crossref] [PubMed]
- Cronin B, Nguyen L, Manecke G, et al. Foreign body located intraoperatively using transesophageal echocardiography. J Cardiothorac Vasc Anesth 2014;28:852-3. [Crossref] [PubMed]
- Cuadra SA, Sales CM, Lipson AC, et al. Misplacement of a vena cava filter into the spinal canal. J Vasc Surg 2009;50:1170-2. [Crossref] [PubMed]
- Danetz JS, McLafferty RB, Ayerdi J, et al. Selective venography versus nonselective venography before vena cava filter placement: evidence for more, not less. J Vasc Surg 2003;38:928-34. [Crossref] [PubMed]
- Kwok PC, Wong WK, Siu KW, et al. Difficult retrieval of a retrievable inferior vena cava filter placed in an inverted orientation. J Vasc Interv Radiol 2006;17:153-5. [Crossref] [PubMed]
- Cappelli F, Vignini S, Baldereschi GJ. ALN inferior vena cava filter upside down rotation with chest caval migration in an asymptomatic patient. J Invasive Cardiol 2010;22:E153-5. [PubMed]
- Milovanovic L, Kennedy SA, Midia M. Procedural and indwelling complications with inferior vena cava filters: frequency, etiology, and management. Semin Intervent Radiol 2015;32:34-41. [Crossref] [PubMed]
- Ahmad I, Yeddula K, Wicky S, et al. Clinical sequelae of thrombus in an inferior vena cava filter. Cardiovasc Intervent Radiol 2010;33:285-9. [Crossref] [PubMed]
- Abtahian F, Hawkins BM, Ryan DP, et al. Inferior vena cava filter usage, complications, and retrieval rate in cancer patients. Am J Med 2014;127:1111-7. [Crossref] [PubMed]
- McAree BJ, O'Donnell ME, Fitzmaurice GJ, et al. Inferior vena cava thrombosis: a review of current practice. Vasc Med 2013;18:32-43. [Crossref] [PubMed]
- Golarz SR, Grimsley B. Use of Wall stent to exclude a thrombosed inferior vena cava filter. Ann Vasc Surg 2010;24:690.e5-7. [Crossref] [PubMed]
- Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med 1998;338:409-15. [Crossref] [PubMed]
- Poudel DR, Pathak R, Karmacharya P, et al. Stuck in the Heart: Embolized Strut Fracture of IVC Filter. Vasc Endovascular Surg 2015;49:93-4. [Crossref] [PubMed]
- Thakur K, Dhawan N, Winchester C, et al. Wire in the heart: fracture and fragment embolization of retrievable inferior vena cava filter into the right ventricle. Case Rep Cardiol 2015;2015:938184.
- Hudali T, Zayed A, Karnath B. A fractured inferior vena cava filter strut migrating to the left pulmonary artery. Respir Med Case Rep 2015;16:3-6. [PubMed]
- Dowell JD, Castle JC, Schickel M, et al. Celect Inferior Vena Cava Wall Strut Perforation Begets Additional Strut Perforation. J Vasc Interv Radiol 2015;26:1510-1518.e3. [Crossref] [PubMed]
- Jehangir A, Rettew A, Shaikh B, et al. IVC filter perforation through the duodenum found after years of abdominal pain. Am J Case Rep 2015;16:292-5. [Crossref] [PubMed]
- Haga M, Hosaka A, Miyahara T, et al. Penetration of an inferior vena cava filter into the aorta. Ann Vasc Dis 2014;7:413-6. [Crossref] [PubMed]
- Kassis C, Kalva SP. Inferior vena cava filter penetration resulting in renal pelvis rupture with urinoma formation. Vasc Endovascular Surg 2013;47:70-2. [Crossref] [PubMed]
- Ishigami N, Nagai T, Arakawa J, et al. Successful Endovascular Removal of a Perforated Inferior Vena Cava Filter Complicated by a Large Retroperitoneal Hematoma: Pitfall of Catheter-Directed Thrombolysis. Int J Angiol 2016;25:70-4. [Crossref] [PubMed]
- Skeik N, McEachen JC, Stockland AH, et al. Lumbar artery pseudoaneurysm caused by a Gunther Tulip inferior vena cava filter. Vasc Endovascular Surg 2011;45:756-60. [Crossref] [PubMed]
- Putterman D, Niman D, Cohen G. Aortic pseudoaneurysm after penetration by a Simon nitinol inferior vena cava filter. J Vasc Interv Radiol 2005;16:535-8. [Crossref] [PubMed]
- Medina CR, Indes J, Smith C. Endovascular treatment of an abdominal aortic pseudoaneurysm as a late complication of inferior vena cava filter placement. J Vasc Surg 2006;43:1278-82. [Crossref] [PubMed]
- Al-Hakim R, Kee ST, Olinger K, et al. Inferior vena cava filter retrieval: effectiveness and complications of routine and advanced techniques. J Vasc Interv Radiol 2014;25:933-9. [Crossref] [PubMed]
- Kuo WT, Tong RT, Hwang GL, et al. High-risk retrieval of adherent and chronically implanted IVC filters: techniques for removal and management of thrombotic complications. J Vasc Interv Radiol 2009;20:1548-56. [Crossref] [PubMed]
- Lynch FC. Removal of a Günther Tulip filter after 3,006 days. J Vasc Interv Radiol 2011;22:337-40. [Crossref] [PubMed]