
Prevalence and risk factors of left atrial thrombus in patients with atrial fibrillation and lower class (IIa) recommendation to anticoagulants
Introduction
Thromboembolic events, particularly stroke, are the most severe complications of atrial fibrillation (AF). The CHA2DS2-VASc score [including congestive heart failure (HF), hypertension (HT), age ≥75 years, diabetes mellitus (DM), stroke/transient ischemic attack (TIA), vascular disease, age 65–75 years, female] is considered a gold standard in the decision process of choosing an anticoagulation treatment strategy.
The previous studies confirmed the role of this scale in predicting thromboembolic risk (1-3). Registry data show that the CHA2DS2-VASc score was better than CHADS2 score in identifying patients ‘risk of thromboembolism (1-3). Kim et al. (4) revealed that the CHA2DS2-VASc score proved well in defining truly low-risk of stroke among Asian patients with AF. However, Willens et al. (5) demonstrated in the multiethnic United States population that the CHA2DS2-VASc is not better than CHADS2 score in identifying the transesophageal echocardiographic risk factors for thromboembolism, such as smoke, sludge, thrombus and abnormal left atrial appendage (LAA) emptying velocity.
On the other hand, it is well known that even AF patients with low CHA2DS2-VASc score may undergo thromboembolic event. According to the current guidelines AF women with 1 point in CHA2DS2-VASc score and men with 0 points require no antithrombotic therapy (6). In women with 2 points and men with 1 point oral anticoagulation therapy (OAT) should be considered (class of recommendation—IIa) but it is not absolutely recommended.
In our work, we tried to assess whether the transesophageal echocardiographic risk for thromboembolism is lower adequately to CHA2DS2-VASc score. Similarly, to many other studies, the left atrial appendage thrombus (LAAT) formation was chosen as a substitute for thromboembolic events.
Therefore, the aim of the present study was to assess the occurrence of LAAT and identify the risk factors of LAAT formation in AF patients and lower class (IIa) recommendation to OAT.
We present the following case series in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/cdt-20-151).
Methods
Study population
We retrospectively evaluated a group of 1,858 patients: 555 with class IIa indication to OAT (IIa group) and 1,303 patients with class I indication (I group). Patients were admitted to three high-reference cardiology departments between 2014 and 2017. All subjects underwent transoesophageal echocardiography (TEE) before cardioversion or ablation.
Baseline assessment
The retrospective data concerning baseline demographic characteristics, the results of clinical evaluation, laboratory tests, echocardiography and treatment strategy at the time of TEE was retrieved from medical records. The clinical evaluation focused on age, gender, cardiovascular risk factors, type of AF and OAT type [vitamin K antagonists (VKAs) vs. non-vitamin K antagonist oral anticoagulants (NOACs)]. The CHA2DS2-VASc score was calculated for each patient in accordance with the current recommendations (6). The information about the comorbidities as part of CHA2DS2VASc scale was retrieved from the medical database according to the International Classification of Diseases—Ninth Revision—Clinical Modification codes and considered only when they were a discharge diagnosis. Paroxysmal AF was defined as AF lasting ≤7 days (6). Patients with a history of persistent AF or with paroxysmal and persistent AF were adjudicated as having non-paroxysmal AF. Laboratory tests included evaluation of renal function [creatinine and estimated glomerular filtration rate (eGFR)] and red blood cells disorders (hemoglobin). eGFR counted from the Modification of Diet in Renal Disease formula (7). Body mass index (BMI) was derived from height and weight.
Transoesophageal echocardiography
TEE was performed in all patients within 48 hours before cardioversion or ablation, most often few hours ahead. General Electric Vivid 7 or E95 ultrasound system (General Electric, Milwaukee, Wisconsin) and Philips EPIQ 7 or iE33 ultrasound system (Philips Medical Systems, Andover, Massachusetts, United States) were used to perform imaging in grade C accredited (according to the Section of Echocardiography of the Polish Cardiac Society) echocardiography laboratories. Focused imaging of the LAA and both of atrial and a continuous sweep from 0 to 180 degrees with short- and long-axis, were a standard TEE acquisition (with standard using of harmonic imaging). The study focused on detecting the thrombus in the LAA. LA thrombus was defined as an echo dense mass in the left atrial (LA) or LAA, with a circular or irregular shape that was not a part of the endocardium or pectinate muscles (8-10). Pulse wave Doppler positioned 1 cm into the orifice of the appendage was used to measure peak LAA emptying velocity (11).
Emptying LAA velocities (LAAV) lower than 20 cm/s were considered diminished. In case of thrombus suspicion, all images were consulted by a second experienced echocardiographer trained in TEE, the cardioversion or ablation procedure was not carried out until another TEE control was conducted after a further period of anticoagulation.
Study endpoint
The primary endpoint was the presence of the left atrial thrombus on TEE.
Statistical analysis
The statistical analysis was performed using Statistica 12.0 (StatSoft, Inc., Tulsa, OK, USA). The distribution and normality of the data were assessed by visual inspection and the Kolmogorov-Smirnov test. Continuous variables were presented as means ± standard deviations (SD) and categorical variables as absolute and relative frequencies (percentages). To analyse the differences between subgroups the student t-test for normally distributed data and Mann-Whitney U-test if the data were not normally distributed were applied. For categorical variables chi square test and Fisher exact test were used. The discriminatory variables (heart failure, hypertension, diabetes mellitus, paroxysmal AF, treatment with VKAs, eGFR <60 mL/min/1.73 m2) were then analysed by univariate and multivariate logistic regression to identify independent predictors of LAAT. A P value of <0.05 was taken to indicate statistical significance.
The study was approved by the Ethics Committee of the Medical University of Warsaw (AKBE/29/2019). The Ethics Committee waived the requirement of obtaining informed consent from the patients to participate in the study.
Results
Baseline characteristics of IIa group
In the IIa group 375 subjects (67.6%) were men with a CHA2DS2-VASc score of 1 point and 180 (32.4%)—women with CHA2DS2-VASc score of 2 points. Table 1 presents the prevalence of particular CHA2DS2-VASc components in this group.

Full table
IIa and I group comparison
The incidence of LAAT was comparable in both IIa and I group: LAAT was confirmed in 30 subjects (5.4%) from IIa group and in 77 subjects (5.9%) from I group. Subjects in IIa group, compared to I group, were: younger (56±8 vs. 62±11 years, P=0.001), more often treated with VKAs [227 (40.9%) vs. 457 (35.1%), P=0.017] and less frequently treated with reduced dosage of NOACs [12 (2.2%) vs. 79 (6.1%), P=0.0003]. The prevalence of treatment with NOACs in both IIa and I group was comparable [298 (53.7%) vs. 760 (58.3%), P=0.065]. Table 2 summarizes the detailed treatment data in both IIa, and I group.

Full table
Only a few patients did not receive any anticoagulation/antiplatelet/combined therapy (IIa group: 3.2%, I group: 4.1%).
Baseline clinical characteristics of both IIa and I group are presented in Table 3.
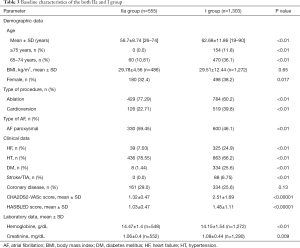
Full table
The relation of LAAT to other clinical data in IIa group
Patients from IIa group with LAAT, in comparison with other subjects from IIa group, presented with lower left ventricular ejection fraction (LVEF) (P=0.03). The prevalence of LAAT in IIa group was also higher in the presence of: heart failure (15.4% vs. 4.7%; P=0.004); diabetes mellitus (25% vs. 5.1%; P=0.014), treatment with VKAs (8.4% vs. 3.4%, P=0.010), eGFR <60 mL/min/1.73 m2 (12.5% vs. 3.6%, P=0.0002) and lower in case of: paroxysmal (in comparison to non-paroxysmal) AF (2.4% vs. 9.8%, P=0.0002), treatment with NOACs (3.4% vs. 7.8%, P=0.021) and in presence of hypertension (3.9% vs. 10.9%; P=0.003).
No significant differences were noted for: age, hemoglobin, creatinine, LAAV, LA diameter, left ventricular diastolic diameter, vascular disease, gender, renal or liver abnormal function, history of bleeding, treatment with reduced NOACs or heparin.
Risk factors of LAAT in IIa group
The multivariate logistic regression revealed the following variables as independent predictors of LAAT in IIa group: treatment with VKAs (OR =2.99; 95% CI: 1.33–6.69; P=0.007), paroxysmal AF (OR =0.26; 95% CI: 0.11–0.62; P=0.002) and eGFR <60 mL/min/1.73 m2 (OR =3.19; 95% CI: 1.42–7.16; P=0.005, Table 4).

Full table
Discussion
Our study focused on the assessment of thromboembolic events in the AF patients with only one additional risk factor (beyond sex) in the CHA2DS2-VASc score. There are only a few studies on this subject, so the data concerning this particular group of patients is uncertain. We showed that the risk of LAAT formation in these patients was comparable to the patients with higher CHA2DS2-VASc score. We realize that this result may be controversial. Strong evidence supports the need for anticoagulation therapy in AF patients with a high risk of thromboembolic events (i.e., CHA2DS2-VASc score ≥2 for women and ≥1 for men) (12-15). OAT is a class I recommendation in patients with a CHA2DS2-VASc risk score of ≥2 in men and ≥3 points in women (6). According to the current guidelines, OAT should also be considered as prevention of thromboembolic events in male AF patients with a CHA2DS2-VASc score of 1 point and women with 2 points, but the class of indication is lower (IIa). While making a decision about OAT therapy in this group of AF patients, other factors, including patient preferences, have to be considered.
The incidence of LAAT in our IIa group was high (5.4%) and comparable to I group. Some other studies suggest clinically relevant risk of stroke even in patients with low CHA2DS2-VASc score. Chao et al. (16), during the follow-up (5.2±4.3 years), reported ischemic stroke in 1,858 patients among 12,935 male AF patients (14.4%) with a CHA2DS2-VASc score of 1 point. The authors demonstrated that not all factors in CHA2DS2-VASc score carry equal risk. The highest risk was seen in those aged 65 to 74 years and those with diabetes mellitus. Also, in our study the prevalence of LAAT in IIa group was higher in the presence of diabetes mellitus (25% vs. 5.1%; P=0.014) and the difference did not apply to age. It may be explained by the fact that the number of patients aged 65–74 years in IIa group was significantly lower compared to group I (11% vs. 36%; P<0.01).
The analysis of the ROCKET-AF clinical trial suggested that the renal impairment is another factor, that could significantly improve the effectiveness of detecting high-risk patients with the CHA2DS2-VASc score (17). Renal dysfunction became a part of a new, CHA2DS2-VASc-based model—the CHA2DS2-VASc-RAF score (R for renal dysfunction, AF for AF type: paroxysmal or non-paroxysmal), which was proposed by Kapłon-Cieślicka et al. (18). Our findings are compatible with that idea—the multivariate logistic regression revealed eGFR <60 mL/min/1.72 m2 as an independent predictor of LAAT in IIa group (OR =3.19; 95% CI: 1.42–7.16; P=0.005).
Our results confirm the advantage of NOACs over VKAs in the reduction of thromboembolic risk. Current guidelines recommend preferring NOACs over warfarin. Many studies showed a lower prevalence of LAAT in AF patients treated with NOACs (19-21). In a large meta-analysis (20) NOACs had a favorable risk-benefit profile, with significant reduction in the prevalence of stroke, intracranial hemorrhage, and mortality, and with similar prevalence of major bleedings compared to warfarin. In the single-center retrospective study, which included AF patients undergoing routine TEE before AF ablation or cardioversion (n=937), the incidence of LAAT was higher in AF patients treated with warfarin (1.55%) compared with AF patients treated with NOACs (0.24%, P=0.047) (20). Similarly, in our study the prevalence of LAAT in IIa group was higher in the AF patients treated with VKAs (8.4% vs. 3.4%, P=0.010) and the multivariate logistic regression revealed that treatment with VKAs is related with 3-fold higher risk of LAAT than the use of NOACs. On the other hand, the study by Gorczyca et al. showed, that NOACs were used for secondary stroke prevention among patients with AF in patients with fewer comorbidities (22).
Higher frequency of LAAT in VKAs treated patients was confirmed by Algarawi et al. (20), who also found non-paroxysmal AF as a significant predictor of LAAT. Similarly, our patients with non-paroxysmal (in comparison to paroxysmal) AF had significantly higher rate of LAAT. These results are consistent with the study by Bertaglia et al. (23), which revealed LAAT in 15/414 patients (3.6%) and almost all of them (14) presented persistent AF. It is worth mentioning that in our study, despite higher prevalence of paroxysmal AF in IIa group (vs. I group: Table 3), the LAAT rate in both groups was comparable.
Finally, in our study HT was one of the factors protecting against LAAT formation. Perhaps the protective role of HT was due to the fact that HT is the most common and also the mildest factor that qualified patients for IIa group.
Limitations
Our study had several limitations. Firstly, the study was a retrospective analysis. Secondly, LAAT is only a surrogate of thromboembolic risk in nonvalvular AF and we did not investigate the true incidence of thromboembolic events. Thirdly, we realize that TEE does not have 100% sensitivity in detecting LAAT. However, in our study TEE examinations were performed by experienced echocardiographers and when a thrombus was suspected, all images were reviewed by a second experienced echocardiographer trained in TEE.
Conclusions
The prevalence of LAAT in AF patients with lower class (IIa) recommendation to anticoagulants was comparable to higher class (I). Treatment with VKAs, along with non-paroxysmal type of AF and eGFR <60 mL/min/1.72 m2 were identified as the strongest predictors of LAAT in IIa group. These risk factors should be considered in clinical decision regarding anticoagulation, despite low thromboembolic risk according to CHA2DS2-VASc score.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors present the study in accordance with the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/cdt-20-151
Data Sharing Statement: The data used to support the findings of this study are available from the corresponding author upon request. Available at http://dx.doi.org/10.21037/cdt-20-151
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/cdt-20-151). IG: paid lectures for Bayer, Boehringer Ingelheim. AKC: personal fees from Bayer, Boehringer Ingelheim, MSD, Pfizer, outside the submitted work. BWK: paid lectures for Bayer, Boehringer Ingelheim, Pfizer. The other authors have no other conflict of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research was conducted in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the Medical University of Warsaw (AKBE/29/2019). The Ethics Committee waived the requirement of obtaining informed consent from the patients to participate in the study. Written informed consents for TEE examination were obtained from each individual.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263-72. [Crossref] [PubMed]
- Mason PK, Lake DE, DiMarco JP, et al. Impact of the CHA2DS2-VASc score on anticoagulation recommendations for atrial fibrillation. Am J Med 2012;125:603.e1-6. [Crossref] [PubMed]
- Jover E, Roldán V, Gallego P, et al. Predictive value of the CHA2DS2-VASc score in atrial fibrillation patients at high risk for stroke despite oral anticoagulation. Rev Esp Cardiol (Engl Ed) 2012;65:627-33. [Crossref] [PubMed]
- Kim TH, Yang PS, Kim D, et al. CHA2DS2-VASc Score for Identifying Truly Low-Risk Atrial Fibrillation for Stroke: A Korean Nationwide Cohort Study. Stroke 2017;48:2984-90. [Crossref] [PubMed]
- Willens HJ, Gómez-Marín O, Nelson K, et al. Correlation of CHADS2 and CHA2DS2-VASc scores with transesophageal echocardiography risk factors for thromboembolism in a multiethnic United States population with nonvalvular atrial fibrillation. J Am Soc Echocardiogr 2013;26:175-84. [Crossref] [PubMed]
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC Endorsed by the European Stroke Organisation (ESO). Eur Heart J 2016;37:2893-962. [Crossref] [PubMed]
- Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999;130:461-70. [Crossref] [PubMed]
- Manning WJ, Weintraub RM, Waksmonski CA, et al. Accuracy of transoesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann Intern Med 1995;123:817-22. [Crossref] [PubMed]
- Aschenberg W, Schlüter M, Kremer P, et al. Transoesophageal two-dimensional echocardiography for the detection of left atrial appendage thrombus. J Am Coll Cardiol 1986;7:163-6. [Crossref] [PubMed]
- Fatkin D, Kelly RP, Feneley MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol 1994;23:961-9. [Crossref] [PubMed]
- Goldberg YH, Gordon SC, Spevack DM, et al. Disparities in emptying velocity within the left atrial appendage. Eur J Echocardiogr 2010;11:290-5. [Crossref] [PubMed]
- Kirchhof P, Ammentorp B, Darius H, et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events-European Registry in Atrial Fibrillation (PREFER in AF). Europace 2014;16:6-14. [Crossref] [PubMed]
- Lip GY, Al‐Khatib S, Cosio F, et al. Contemporary Management of Atrial Fibrillation: What Can Clinical Registries Tell Us About Stroke Prevention and Current Therapeutic Approaches? J Am Heart Assoc 2014;3:e001179. [Crossref] [PubMed]
- Lip GY, Laroche C, Ioachim PM, et al. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one-year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur Heart J 2014;35:3365-76. [Crossref] [PubMed]
- Potpara TS, Polovina MM, Licina MM, et al. Reliable identification of "truly low" thromboembolic risk in patients initially diagnosed with "lone" atrial fibrillation: the Belgrade atrial fibrillation study. Circ Arrhythm Electrophysiol 2012;5:319-26. [Crossref] [PubMed]
- Chao TF, Liu CJ, Wang KL, et al. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol 2015;65:635-42. [Crossref] [PubMed]
- Piccini JP, Stevens SR, Chang Y, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once‐daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation 2013;127:224-32. [Crossref] [PubMed]
- Kapłon-Cieślicka A, Budnik M, Gawałko M, et al. Atrial fibrillation type and renal dysfunction as important predictors of left atrial thrombus. Heart 2019;105:1310-5. [Crossref] [PubMed]
- Wyrembak J, Campbell KB, Steinberg BA, et al. Incidence and Predictors of Left Atrial Appendage Thrombus in Patients Treated With Nonvitamin K Oral Anticoagulants Versus Warfarin Before Catheter Ablation for Atrial Fibrillation. Am J Cardiol 2017;119:1017-22. [Crossref] [PubMed]
- Alqarawi W, Birnie DH, Spence S, et al. Prevalence of left atrial appendage thrombus detected by transoesophageal echocardiography before catheter ablation of atrial fibrillation in patients anticoagulated with non-vitamin K antagonist oral anticoagulants. Europace 2019;21:48-53. [Crossref] [PubMed]
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62. [Crossref] [PubMed]
- Gorczyca I, Michalska A, Chrapek M, et al. Non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation in secondary stroke and systemic embolism prevention. Cardiol J 2019. [Epub ahead of print]. [PubMed]
- Bertaglia E, Anselmino M, Zorzi A, et al. NOACs and atrial fibrillation: Incidence and predictors of left atrial thrombus in the real world. Int J Cardiol 2017;249:179-83. [Crossref] [PubMed]


