Comparison of prognosis between surgical resection and transarterial chemoembolization for patients with solitary huge hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the second most lethal malignancy after pancreatic cancer (1), being responsible for 745,500 deaths worldwide in 2012 and ranking as the second and sixth most common cause of cancer among men and women, respectively (2). According to the current standards of HCC management, treatments are classified as either curative or non-curative (3). Curative treatments include surgical resection (SR), liver transplantation, and local ablation therapies, which could provide a median overall survival (OS) of over five years for HCC patients after treatment (1). Non-curative treatments include transarterial chemoembolization (TACE), transarterial radioembolization, external beam radiotherapy, and systemic therapy (e.g., molecular target therapy, immune-based therapy). For patients with huge (>10 cm in diameter) HCC, local ablation is unfeasible because the tumor size exceeds physical limitation. Liver transplantation is also inapplicable given that the size exceeds the current criteria (4-6). Consequently, SR and TACE are more popular alternatives in this clinical context.
However, HCC patients with a larger tumor size displayed a higher incidence of vascular invasion and satellite nodules relative to those with a smaller tumor (7). Considering that vascular invasion is a major risk factor for tumor recurrence and poor OS after surgery (8-11), the indication and treatment efficacy of SR for patients with huge HCC is a topic of debate. Generally, Asian countries have adopted more liberal application of SR for HCC (12,13), whereas the current guidelines for the management of HCC set by the European Association for the Study of the Liver (EASL) limited SR to patients with solitary HCC, well-preserved liver function, normal portal pressure and serum bilirubin levels, good performance status, and without extra-hepatic metastasis or vascular invasion (14). However, there is a growing body of evidence suggesting that SR could yield favorable outcomes for HCC patients with portal hypertension, size exceeding 5 cm, multiple tumors, macroscopic vascular invasion, or even for those presenting with solitary extra-hepatic organ involvement (7,15-22). Another widely-applied classification system in Asia, Hong Kong Liver Cancer (HKLC), adopted more liberal criteria to SR (23).
Theoretically speaking, in the absence of distant metastasis and major vascular invasion, a clear-margin resection seems to serve as a curative therapy regardless of tumor size and number (16,21). We hypothesized that, for patients with solitary huge HCC in addition to well-preserved liver function, SR may still provide a survival advantage over the guideline-endorsed treatment TACE. To test this hypothesis, the long-term prognoses between SR and TACE for patients with single huge HCC were compared.
Methods
Patients
A prospectively conducted and retrospectively analyzed cohort study was conducted. The diagnosis of HCC was established according to the criteria set forth by the American Association for the Study of Liver Disease (AASLD) (24). All patients newly diagnosed with HCC at Taipei General Veterans Hospital were discussed to determine treatment strategy by a weekly-convened, multi-discipline HCC panel meeting attended by an oncologist, gastroenterologist, surgeon, radiologist, pathologist, onco-radiologist, and nursing personnel (25,26). Following the meeting, treatment modality decision was shared with the patient and the physician after discussing the risks, benefits, complications, efficacies of the currently available treatments, and the multidisciplinary experts’ recommendations. The demographic characteristics, treatment modalities, and prognoses of all patients with newly diagnosed HCC were prospectively recorded in the database for the multidisciplinary committee.
In this study, the inclusion criteria were as follows: (I) treatment-naïve HCC; (II) solitary tumor with size ≥10 cm; (III) well-preserved liver function with Child-Pugh grade A or B; (IV) absence of major portal branch invasion by computed tomography scan or magnetic resonance imaging; (V) absence of distant metastases; and (VI) SR or TACE as the first treatment for HCC.
The study was conducted in accordance with the Declaration of Helsinki and current ethical guidelines. It was approved by the Institutional Review Board (IRB) of the Taipei Veterans General Hospital. Informed consent was obtained before the patient underwent SR or TACE. Patient information was anonymized prior to the initiation of this study.
Statistical analysis
The primary endpoint of this study was OS. All patients were followed up until either their final hospital visit, death, or December 31, 2017. Student’s t-test and Mann-Whitney U test were adopted for parametric and non-parametric distributed continuous variables, respectively. χ2 test and Fisher’s exact test were applied for parametric and non-parametric nominal variables, respectively. Kaplan-Meier survival analysis was adopted for estimating OS after therapy. Cox proportional hazards model was employed to determine the factors associated with OS. Factors with a P value <0.05 in the univariate analysis were enrolled to multivariate analysis by a forward stepwise Cox’s regression model.
We were aware that baseline demographic differences may confound OS analysis results. Therefore, a propensity-matched scoring (PSM) method was used to adjust for differences of demographic characteristics and tumor factors between the two groups of patients as previously described (27,28). Subsequently, a one-to-one match between the SR and TACE groups was obtained using the nearest-neighbor matching method. Survival analysis was repeated to compare the OS between SR and TACE amended from these confounding factors. A two-tailed P value <0.05 was defined as statistically significant. All statistical analyses were performed by IBM SPSS Statistics for Windows, Version 21.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline demographic characteristics
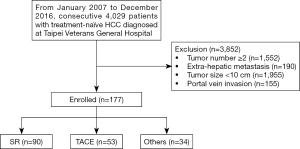
From January 2007 to December 2016, a total of 4,029 patients were diagnosed with HCC in our center. Among these, 177 presented with a solitary huge HCC with a characteristic tumor size ≥10 cm, and without main portal branch invasion or distant metastases. Ninety of these patients underwent SR, 53 patients underwent TACE, and the remaining 34 patients received another treatment such as radiotherapy, chemotherapy, or best supportive treatments (Figure 1).
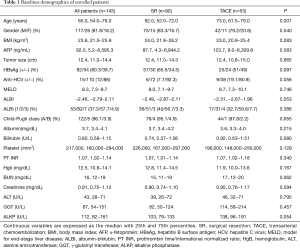
As shown in Table 1, patients who underwent SR were younger than their TACE counterparts (P=0.001). Regarding the viral etiology, although results were not statistically significant, patients who had undergone SR displayed a trend of higher rate of hepatitis B virus (HBV) infection and lower rate of antibodies against hepatitis C virus (HCV) in sera relative to those treated with TACE. Otherwise, gender, tumor size, and liver functional reserve did not differ significantly between the two groups.
Full table
Factors related to OS
There were two mortality cases within 30 days after TACE and no cases in the SR group. The 90 days mortality were six and two cases in the TACE group and in the SR group respectively. Following a median follow-up period of 17.0 (interquartile range IQR 7.7–45.6) months, 83 patients had died. As shown in Figure 2A, the 1-, 2-, 3-, and 5-year cumulative OS rate for patients who underwent SR and TACE were 78.1% vs. 45.1%, 67.5% vs. 27.6%, 58.3% vs. 20.7%, and 44.7% vs. 11.7%, respectively. The median OS was 55.7 (95% confidence interval CI: 23.4−88.1) months and 11.6 (95% CI: 8.5−14.6) months for patients receiving SR and TACE, respectively. Patients treated with SR displayed better survival relative to those treated with TACE (P<0.001).
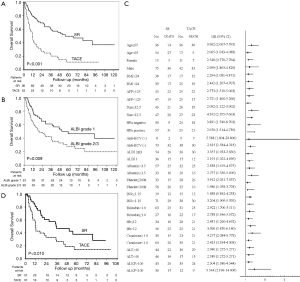
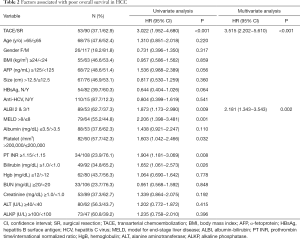
A univariate analysis disclosed that TACE, low platelet count, prothrombin time (PT) international normalized ratio (INR) ≥1.15, serum bilirubin ≥1.0 mg/dL, and albumin-bilirubin (ALBI) grade 2 or 3 (Figure 2B) were associated with poorer OS for patients with a solitary huge HCC (Table 2). Multivariate analysis showed that TACE (HR: 3.515, 95% CI: 2.202−5.610, P<0.001) and ALBI grade 2 or 3 (HR: 2.181, 95% CI: 1.343−3.543, P=0.002) were the independent factors predicting poorer OS. Subgroup analysis further revealed that, in the majority of patient subgroups, patients who underwent SR had a better OS than those who had received TACE, with the exception of female patients and patients with a PT INR ≥1.15 (Figure 2C).
Full table
Comparison of OS between SR and TACE after PSM analysis
Because the baseline demographic characteristics were dissimilar between patients who underwent SR of TACE, a PSM analysis was performed to adjust for the differences between both of these groups. Subsequently, 74 patients (37 per group) were selected. As shown in Table 3, following the PSM, baseline demographics between both groups were well-matched, and survival analysis still indicated that SR resulted in better OS than did TACE (P=0.010; Figure 2D).
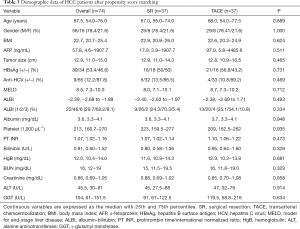
Full table
Comparison of OS between SR and TACE stratified by ALBI grade
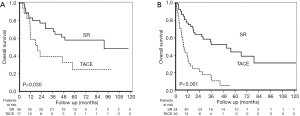
As treatment modality and ALBI grade were the independent factors correlated with poorer OS according to multivariate analysis, we further compared the prognoses between patients receiving SR or TACE by stratification by ALBI grade. In our cohort, although most patients were in Child-Pugh class A liver functional reserve (96.1%), the proportion of ALBI grade was 37.3%, 57.7%, and 4.9% for grade 1, 2, and 3, respectively. The distribution of ALBI grades in SR and TACE groups did not present statistically significant differences (P=0.386). Survival analysis showed that SR had better survival than TACE, both in terms of ALBI grade 1 (Figure 3A, P=0.030) and ALBI grade 2 or 3 (Figure 3B, P<0.001). For patients with ALBI grade 1, the 1-, 2-, 3-, and 5-year cumulative OS rate for patients having undergone SR vs. TACE were 83.2% vs. 58.8%, 77.3% vs. 39.2%, 71.1% vs. 39.2%, and 58.0% vs. 24.5%, respectively. For those with ALBI grade 2 or 3, the 1-, 2-, 3-, and 5-year cumulative OS rate for patients having undergone SR vs. TACE were 77.1% vs. 38.1%, 64.1% vs. 21.8%, 52.2% vs. 14.5%, and 43.8% vs. 5.4%, respectively.
Factors of OS and recurrence-free survival in SR group
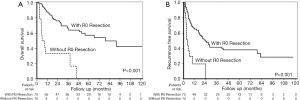
Among the 90 patients who underwent SR, 75 patients (83.3%) reached R0 resection (no cancer cells were found in surgical margin, and tumor was completely resected). Pathological examination showed that 9 (10%) patients had macrovascular invasion, and 79 patients (87.8%) had microvascular invasion in their surgical specimen. When stratified by the status of R0 resection, the cumulative 1-, 2-, 3-, and 5-year cumulative OS rates were 87.9% vs. 33.3%, 76.1% vs. 33.3%, 65.9% vs. 33.3% and 57.3% vs. 0% in the R0 resection group and in the non-R0 resection group, respectively (Figure 4A, P<0.001).
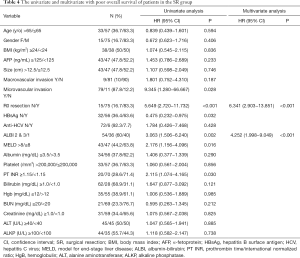
As shown in Table 4, a multivariate analysis disclosed the independent prognostic factors to poorer OS included ALBI grade 2 or 3 (HR: 4.252, 95% CI: 1.998–9.049, P<0.001) and no R0 resection (HR: 6.341, 95% CI: 2.903−13.851, P<0.001).
Full table
Besides, 46 patients developed tumor recurrence after tumor resection. The median recurrence time was 5.7 (IQR 3.2−14.4) months. The median recurrence free survival (RFS) was 19.4 (IQR 13.1−25.7) months. The cumulative 1-, 2-, 3-, and 5-year cumulative RFS rates were 65.3% vs. 19.6%, 49.4% vs. 0%, 41.5% vs. 0% and 38.1% vs. 0%, respectively in the R0 resection group and in the non-R0 resection group, respectively (Figure 4B, P<0.001).
A multivariate analysis showed that age >65 years (HR: 2.004, 95% CI: 1.106−3.636, P=0.022), serum alpha-fetoprotein (AFP) level ≥125 ng/mL (HR: 1.772, 95% CI: 1.024−3.067, P=0.041), ALBI grade 2 or 3 (HR: 5.055, 95% CI: 2.687−9.511, P<0.001) and no R0 resection (HR: 5.458, 95% CI: 2.644−11.264, P<0.001) were the independent factors predicting poorer RFS (Table 5).
Full table
Discussion
Our study reports several major findings. First, for patients with a solitary huge HCC, SR yielded a better long-term OS than did TACE, which was further substantiated by multivariate analysis, PSM analysis, and subgroup analysis. SR was found to be safe and may be served as the front-line treatment modality for such patients given that they are not contraindicated for the operation. Second, ALBI grade possessed a discriminatory capability to predict prognosis for patients with solitary huge HCC. It suggested that liver functional reserve still played a crucial role in determining the outcomes of patients with huge HCC, and it could provide an important reference to predict prognosis in this clinical setting.
Regarding evaluation of SR’s suitability for patients with HCC, Eastern and Western medicinal perspectives might differ. For the Eastern world, large size, microscopic portal vein invasion, and presence of clinically significant portal hypertension (CSPH) do not alter surgeons’ judgement on carrying out resection once a clear-cut margin is attainable and liver function is well-reserved (12,16,29,30). Conversely, SR is discouraged in such clinical contexts by their Western counterparts (6). The present study showed that, for patients with solitary huge HCC in which the tumor size exceeded the Milan criteria, the median OS and 5-year cumulative OS rate in those having undergone SR were 55.7 months and 44.7%, respectively. The results appeared satisfactory when compared to the previous results (31-33), and they were significantly better than those for patients who underwent TACE. Although major vascular invasion was excluded from our study, microscopic vascular invasion was expected in the majority of enrolled patients due to the tumor size surpassing 10 cm in diameter (7). Indeed, by pathological examination, macroscopic and microscopic vascular invasion were found in 10.0% and 87.8% of the patients in the SR group, respectively. However, both macroscopic and microscopic vascular invasion were not associated with poorer OS and RFS by multivariate analysis. By stead, ALBI grade and R0 resection could determine the outcomes of patients with solitary huge HCC after SR. It implied that microscopic vascular invasion did not appear to hinder SR effectiveness relative to TACE if liver function was well preserved and R0 resection could be achieved. However, more prospective studies are warranted to elucidate the impact of microscopic vascular invasion on the outcomes of patients with solitary huge HCC and on the selection of the treatment modality for these patients.
The HKLC system classifies patients with HCC size exceeding 5 cm, numbers less than 3, and good liver functional reserve (Child-Pugh class A) as IIb (23). In their cohort, SR and TACE comprised the most common treatment modalities in this tumor stage. Moreover, SR could provide a survival benefit relative to TACE. Hence, SR is the recommended treatment in this clinical setting. Our study further validated that, even for patients with HCC tumor size exceeding 10 cm, SR could proffer better survival than could TACE. A further liberal step beyond current guideline-charted territory of SR may be justified.
Conventional assessment of liver reserve, the Child-Pugh classification, is not sufficiently accurate because the extents of ascites and encephalopathy were subjective variables (34). Furthermore, Child-Pugh class was originally designed for cirrhotic patients, and its use in patients with HCC prognosis may be inaccurate (35). Accordingly, Johnson recently proposed that ALBI scores, which incorporated both serum albumin and bilirubin levels, could provide a simple, objective, and evidence-based method for the evaluation of liver function for patients with liver cirrhosis or HCC (36). Moreover, it has been validated to accurately predict the prognoses of patients with HCC across different tumor stages and treatment modalities (34-41).
In our cohort, although most patients fell in the Child-Pugh class A (96.1%), only 37.3% of the patients were classified as ALBI grade 1. For these patients, ALBI grade still presented as an excellent predictor of prognosis. For patients with ALBI grade 1, the 5-year OS rate after SR was as high as 58.0%. This suggests that, for patients with solitary huge HCC and ALBI grade 1 liver reserve, SR could be encouraged as the front-line treatment rather than be restricted to guideline-endorsed treatment, given that resection is highly likely to yield favorable long-term outcomes.
By the current concept, SR is a curative treatment modality while TACE is regarded as a non-curative local regional therapy for the treatment of HCC. Nevertheless, for patients with solitary huge HCC, substantial patients received TACE because of the concerns of safety and treatment efficacy of SR due to large tumor burden and the presence of microvascular invasion. In our cohort, no patient died within 30 days after the operation in the SR group. Moreover, the 90-day morality rates were also lower in the SR group when compared to that in the TACE group. Besides, the OS rate was also better in the SR group even given in the fact that most of the patients who underwent SR had microscopic invasion. In suggested that SR could be served as the front-line therapy for solitary huge HCC if the patients with a well-preserved liver function and R0 resection could be achieved.
There were some limitations to our study. First, the study was retrospective and, second, our institution is a tertiary referral medical center. Hence, selection bias cannot be ruled out. Third, SR is—at minimum—a partially operator-dependent intervention. Our institutional experience may deviate from that of other institutions. Fourth, approximately 60% of patients had chronic HBV infection. In Taiwan, HBV is the major viral factor attributed to HCC carcinogenesis. On the other hand, chronic HCV infection accounts for the majority of HCC in Western countries, as patients with HBV-induced HCC might have a lower rate of cirrhosis and better liver functional reserve relative to HCV-related HCC (42,43). Conversely, regarding tumor factors, patients with HBV-related HCC seemed to have a higher rate of harboring gene signatures in the proliferation class, which was associated with poor prognosis compared to HCV-related HCC (1). Consequently, whether different initial carcinogenetic factors resulted in identical disease course and prognosis remains to be elucidated. Hence, generalizations of our results should remain conservative, with external validation from other cohorts highly encouraged. Fifth, several new treatment modalities, such as drug-eluting beads TACE, selective internal radiation therapy, molecular target therapy, and immune checkpoint inhibitors have been applied for patients with large HCC (1). Further prospective studies are warranted to assess the treatment efficacy and prognosis of these new treatment modalities for patients with solitary huge HCC.
In conclusion, for patients with solitary huge HCC, absence of major portal vein invasion, and no extra-hepatic metastasis, SR resulted in better survival than did TACE.
Acknowledgments
Part of this study was presented as a poster presentation at the 2018 annual meeting of the European Association for the Study of the Liver, Paris, France, April 11−15, 2018. Writing Assistance: American Manuscript Editors.
Funding: This work was supported by grants from the Ministry of Science and Technology of Taiwan (MOST 106-2314-B-038-030, MOST 108-2314-B-075-049-MY3), and Taipei Veterans General Hospital (V107C-113, V108C-050, V107D36-003-MY2-2, VGHUST108-G6-1-2, Center of Excellence for Cancer Research MOHW107-TDU-B-211-114019, and Big Data Center).
Footnote
Conflicts of Interest: Chien-Wei Su: Speakers’ bureau: Gilead Sciences, Bristol-Myers Squibb, AbbVie, Bayer, and Roche. Advisory arrangements: Gilead Sciences. Other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki and current ethical guidelines. It was approved by the Institutional Review Board (IRB) of the Taipei Veterans General Hospital. Informed consent was obtained before the patient underwent SR or TACE.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-62. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Kamo N, Kaido T, Yagi S, et al. Liver Transplantation for Intermediate-Stage Hepatocellular Carcinoma. Liver Cancer 2018;7:179-89. [Crossref] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Kluger MD, Salceda JA, Laurent A, et al. Liver resection for hepatocellular carcinoma in 313 Western patients: Tumor biology and underlying liver rather than tumor size drive prognosis. J Hepatol 2015;62:1131-40. [Crossref] [PubMed]
- Su CW, Chau GY, Hung HH, et al. Impact of steatosis on prognosis of patients with early-stage hepatocellular carcinoma after hepatic resection. Ann Surg Oncol 2015;22:2253-61. [Crossref] [PubMed]
- Chen PC, Fang KC, Kao WY, et al. Outcomes of resection surgery for hepatocellular carcinoma: Does size matter? J Chin Med Assoc 2018;81:89-91. [Crossref] [PubMed]
- Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol 2018;69:1284-93. [Crossref] [PubMed]
- Hwang S, Lee YJ, Kim KH, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: Single-institution experience with 2558 patients. J Gastrointest Surg 2015;19:1281-90. [Crossref] [PubMed]
- Surveillance group. Staging group; Surgery group; Local ablation group; TACE/TARE/HAI group; Target therapy/systemic therapy group; Radiotherapy group; Prevention group; Drafting group. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J Formos Med Assoc 2018;117:381-403.
- Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver Cancer 2018;7:235-60. [Crossref] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Yamamoto S, et al. Surgical treatment of hepatocellular carcinoma associated with hepatic vein tumor thrombosis. J Hepatol 2014;61:583-8. [Crossref] [PubMed]
- Ishizawa T, Hasegawa K, Aoki T, et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology 2008;134:1908-16. [Crossref] [PubMed]
- Chang WT, Kao WY, Chau GY, et al. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery 2012;152:809-20. [Crossref] [PubMed]
- Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg 2013;257:929-37. [Crossref] [PubMed]
- Roayaie S, Jibara G, Tabrizian P, et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology 2015;62:440-51. [Crossref] [PubMed]
- Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329-40. [Crossref] [PubMed]
- Vitale A, Burra P, Frigo AC, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol 2015;62:617-24. [Crossref] [PubMed]
- Cucchetti A, Cescon M, Golfieri R, et al. Hepatic venous pressure gradient in the preoperative assessment of patients with resectable hepatocellular carcinoma. J Hepatol 2016;64:79-86. [Crossref] [PubMed]
- Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology 2014;146:1691-700.e3. [Crossref] [PubMed]
- Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Hsieh WY, Chen PH, Lin IY, et al. The impact of esophagogastric varices on the prognosis of patients with hepatocellular carcinoma. Sci Rep 2017;7:42577. [Crossref] [PubMed]
- Fang KC, Kao WY, Su CW, et al. The prognosis of single large hepatocellular carcinoma was distinct from Barcelona Clinic Liver Cancer Stage A or B: The role of albumin-bilirubin grade. Liver Cancer 2018;7:335-58. [Crossref] [PubMed]
- D'Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-81. [Crossref] [PubMed]
- Hung HH, Chiou YY, Hsia CY, et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin Gastroenterol Hepatol 2011;9:79-86. [Crossref] [PubMed]
- Chang CY, Hsieh WY, Chau GY, et al. Esophageal varices are not predictive of patient prognosis after surgical resection of hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2018;30:1368-77. [Crossref] [PubMed]
- Hsu CY, Liu PH, Hsia CY, et al. Surgical resection is better than transarterial chemoembolization for patients with hepatocellular carcinoma beyond the Milan criteria: A prognostic nomogram study. Ann Surg Oncol 2016;23:994-1002. [Crossref] [PubMed]
- Yamashita Y, Taketomi A, Shirabe K, et al. Outcomes of hepatic resection for huge hepatocellular carcinoma (>/= 10 cm in diameter). J Surg Oncol 2011;104:292-8. [Crossref] [PubMed]
- Zhu SL, Zhong JH, Ke Y, et al. Efficacy of hepatic resection vs transarterial chemoembolization for solitary huge hepatocellular carcinoma. World J Gastroenterol 2015;21:9630-7. [Crossref] [PubMed]
- Shrager B, Jibara GA, Tabrizian P, et al. Resection of large hepatocellular carcinoma (>/=10 cm): a unique western perspective. J Surg Oncol 2013;107:111-7. [Crossref] [PubMed]
- Kao WY, Su CW, Chiou YY, et al. Hepatocellular carcinoma: nomograms based on the albumin-bilirubin grade to assess the outcomes of radiofrequency ablation. Radiology 2017;285:670-80. [Crossref] [PubMed]
- Hiraoka A, Kumada T, Tsuji K, et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: A multicenter analysis. Liver Cancer 2019;8:121-9. [Crossref] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-The ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- Chen PC, Chiu NC, Su CW, et al. Albumin-bilirubin grade may determine the outcomes of patients with very early stage hepatocellular carcinoma after radiofrequency ablation therapy. J Chin Med Assoc 2019;82:2-10. [Crossref] [PubMed]
- Chen PH, Hsieh WY, Su CW, et al. Combination of albumin-bilirubin grade and platelets to predict a compensated patient with hepatocellular carcinoma who does not require endoscopic screening for esophageal varices. Gastrointest Endosc 2018;88:230-239.e2. [Crossref] [PubMed]
- Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017;66:338-46. [Crossref] [PubMed]
- Chan AW, Kumada T, Toyoda H, et al. Integration of albumin-bilirubin (ALBI) score into Barcelona Clinic Liver Cancer (BCLC) system for hepatocellular carcinoma. J Gastroenterol Hepatol 2016;31:1300-6. [Crossref] [PubMed]
- Lee PC, Chen YT, Chao Y, et al. Validation of the albumin-bilirubin grade-based integrated model as a predictor for sorafenib-failed hepatocellular carcinoma. Liver Int 2018;38:321-30. [Crossref] [PubMed]
- Kao WY, Su CW, Chau GY, et al. A comparison of prognosis between patients with hepatitis B and C virus-related hepatocellular carcinoma undergoing resection surgery. World J Surg 2011;35:858-67. [Crossref] [PubMed]
- Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology 2019;156:477-491.e1. [Crossref] [PubMed]