
Xuezhitong capsule, an extract of Allium macrostemon Bunge, exhibits reverse cholesterol transport and accompanies high-density lipoprotein levels to protect against hyperlipidemia in ApoE−/− mice
Introduction
Hyperlipidemia, subsequent atherosclerosis, and a series of cardiovascular diseases (CVDs) are major public health challenges due to their extremely high morbidity and mortality worldwide (1,2). Clinical patients who have abnormal lipid homeostasis also typically suffer from atherosclerotic heart diseases and hypertrophy. Laboratory data have shown that the rate of hyperlipidemia in response to lipid metabolism disorder is elevated in ApoE−/− mice, which provide compelling evidence that ApoE−/− mice treated with Western diet have a high probability to suffer from hyperlipidemia (3). According to other evidence, lipid metabolism disorder is demonstrated by increased total cholesterol (TC), triglyceride (TG), and low-density lipoprotein (LDL) cholesterol levels and decreased plasma high-density lipoprotein (HDL) cholesterol levels in the blood (1). These disorders consistently exacerbate the situation.
During the development of hyperlipidemia, most of the cholesterol syntheses occur in the liver (2). The rate-limiting enzyme used for cholesterol synthesis is 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoAR). A total of 70% of cholesterol in human serum are carried by LDL and VLDL, and most of the circulating LDLs are cleared in the liver via LDL receptor (LDLR) pathway. LDLR increases LDL degradation by mediating LDL uptake and is important in maintaining a relatively constant plasma LDL level. Reverse cholesterol transport (RCT) is the process of removing excess cholesterol from peripheral tissues, such as macrophages by HDL, and these cholesterols ultimately metabolized or excreted by the liver (4,5). The ATP-binding cassette transporter A1 (ABCA1) and scavenger receptor class B type 1 (SRB1) are two important functional proteins in the RCT process. ABCA1 mediates the transport of intracellular phospholipids and free cholesterol to the poor or fat-free apolipoprotein A I (ApoA1), thereby promoting HDL production, initiating the RCT process, and playing an important role in the removal of excess lipids. SRBI has been identified as a functional HDL receptor and acknowledged to a potential new target for cardiovascular drugs (6). Therefore, RCT can be enhanced by increasing SRBI expression levels, reducing atherosclerotic lesions by accelerating clearance of extra-cholesterol esters in extrahepatic tissue cells and promoting cholesterol reduction in early atherosclerotic lesions. Thus, drugs that increase ABCA1 and SRBI expression can limit the occurrence of hyperlipidemia by accelerating the RCT process and promoting the elimination of excess lipid accumulation. Clinical practices have revealed that some lipid-lowering drugs reduce the LDL cholesterol levels, thereby promoting cardiovascular protection, of statins, fibrates, niacin, bile acid sequestrants, and cholesterol absorption inhibitors (7,8). However, due to undesirable side effects, residual cardiovascular risk or, high prices, and patient preference, effective alternative drugs with low toxicity should be explored to ameliorate hyperlipidemia. In this study, we focused on the antihyperlipidemic effects of Xuezhitong capsule (XZT) on hyperlipidemia in response to lipid metabolism disorders. The components of XZT are derived from Allium macrostemon Bunge, which is also known as Xie Bai, has been used for abnormal lipid homeostasis treatment by maintaining the metabolic balance. Xie Bai, is an ancient Chinese medicinal plant that is widely used to treat CVDs in China, and exhibits immune-enhancing, antitumor, antioxidant, and antiasthmatic properties. Many studies have demonstrated that Xie Bai mitigates acute myocardial ischemia and maintains metabolic balance by regulating amino acid metabolism and controlling the change in energy metabolism (9), in which the enhanced vasodilation mediated by PKA/NO pathway (10), and ameliorated depression via its mechanism is connected with its protective function on neurogenesis and brain derived neurotrophic factor (BDNF) release (11). However, the mechanism underlying the antihyperlipidemic effect of XZT remains largely unknown. This study is the first to elucidate the mechanism underlying the effect of XZT in ApoE−/− mice hyperlipidemia. To the best of our knowledge, XZT exhibits antihyperlipidemic effects that ameliorate high fat diet-induced dyslipidemia of ApoE−/− mice. The mechanisms are closely correlated with RCT, which increases the HDL effects to mitigate hyperlipidemia that is largely enhanced by XZT administration.
Methods
Drugs
XZT provided by Dongfang Pharmaceutical Co., Ltd. (Jilin, China). Xuezhikang capsule (XZK) was obtained from Beijing WBL Peking University Biotechnology Co. Ltd. (Beijing, China). Atorvastatin (ATO) was purchased from Pfizer Pharmaceuticals Ltd. (New York, USA). Before study, they were freshly prepared in 0.9% normal saline.
Animals and in vivo pharmacological treatment
All animal care and experimental protocols were approved by the Institutional Animal Care and Use Committee of Chinese Academy of Medical Sciences and Peking Union Medical College. The treatment scheme of mice is concisely described in Figure S1. A total of 200 animals were used in the experiments. Male ApoE−/− C57BL/6J mice (6-week-old, 18–20 g body weight) were randomly assigned to 9 groups. The control group (WT; n=20) comprised C57BL/6J mice injected i.g. with normal saline (solvent for XZT capsules). The model group (ApoE−/−, n=20) comprised mice treated with high-fat diet (1.25% cholesterol, 0% cholate; Research Diets) for 34 weeks. The XZT group (XZT, n=60) comprised mice treated with XZT at doses of 400, 800, and 1,600 mg/kg i.g. every day for different dose groups. The XZT and ATO (atorvastatin) cotreatment groups (XZT + ATO, n=40) comprised mice treated with XZT at a dose of 800 mg/kg i.g. every day, followed by ATO at a dose of 3 or 6 mg/kg i.g. every day for different dose groups. The positive control group (ATO/XZK group, n=60) comprised mice treated with ATO at a dose of 3 or 6 mg/kg and XZK capsules at a dose of 180 mg/kg i.g., every day for different groups. At week 34, serum, plasma, liver, abdominal fat, and gastrocnemius tissues were obtained to investigate the mechanism underlying hyperlipidemia in XZT regulation in ApoE−/− mice.
Serum lipid determination
Serum lipid levels were detected using commercial enzymatic kits (Jiancheng Bioengineering Institute, Nanjing, China) for markers containing TG, TC, LDL, and HDL. To determine the serum lipids, we fasted mice (n=5) 12 h at the 34th week of administration, collected blood from the abdominal aorta, and centrifuged at 3,000 ×g for 10 min, and separated and stored serum at −80 °C for lipid detection.
Histological analysis
Mice were perfused with saline through the left ventricle. The aortic arch tissue was collected immediately and fixed in 4% paraformaldehyde solution overnight. Then paraffin sections (5 µm) were taken for hematoxylin-eosin (HE) and Oil Red O staining according to standard procedures. The pathological results were observed under a light microscope.
Lipid metabolism enzyme determination in plasma, liver, abdominal fat, and gastrocnemius
After administration for 34 weeks, blood was collected from the abdominal aorta to prepare plasma. Liver, abdominal fat, and gastrocnemius muscle were also isolated. The free fatty acid (FFA), oxidized LDL (ox-LDL), visfatin, acyl coenzyme A: cholesterol acyltransferase (ACAT), lecithin cholesterol acyltransferase (LCAT), ApoA1, ApoB, LPL, HL levels in plasma and liver were quantified by ELISA kit (Expandbio, Beijing, China). Similarly, the expression levels of fatty acid synthase (FAS), HSL, TGH were detected in the liver, gastrocnemius and adipose tissue. Moreover, liver LDLR, HMG-CoAR, ABCA1 and SRB1 were also determined in the same way.
Statistical analysis
Data were expressed as mean ± SD. The statistical significance of differences was evaluated using Student’s t-test, and significance was set at P<0.05. One-way ANOVA coupled with post hoc test was conducted to determine the significance for multiple comparisons. Calculations were performed using SPSS (version 17.0) statistical software.
Results
XZT inhibits the development of hyperlipidemia in ApoE−/− mice
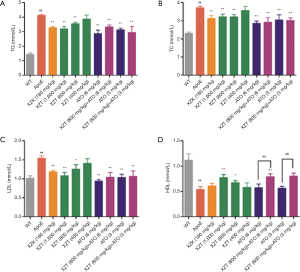
To examine the anti-hyperlipidemic effect of XZT, serum lipid levels were detected and histological changes in the aorta were analysis. As shown in Figure 1, the TG, TC, and LDL levels in ApoE−/− mice significantly increased (P<0.01), whereas HDL levels showed considerable decrease compared with WT mice (P<0.01). These data indicated that the hyperlipidemia model was successfully established. However, XZT (1,600 and 800 mg/kg) administration for 34 weeks significantly reduced the TG, TC, and LDL levels (P<0.05) and increased the HDL levels (P<0.05) compared with ApoE−/− mice. Serum HDL levels showed significant increase in mice cotreated with XZT (800 mg/kg) and ATO (6 mg/kg) compared with those treated with ATO (6 mg/kg) alone (P<0.05). These results indicate that XZT improves the high fat diet-induced lipid abnormalities in atherosclerosis-prone mice.
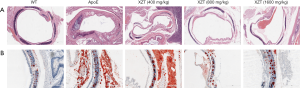
HE and Oil Red O staining indicated that the endangium of the WT mice was structured and the smooth muscle cells had a consistent and regular size. No obvious atherosclerotic plaque was observed. Conversely, there are typical atherosclerotic plaque formation, showing lipid deposition, cholesterol crystallization and fibrous plaque in ApoE−/− mice. XZT reduced the plaque area and ameliorated cholesterol crystallization. Moreover, fibrous plaque was thickened to stabilize the plaque in response to XZT treatment (Figure 2).
XZT improved high fat diet-induced lipid metabolism in ApoE−/− mice
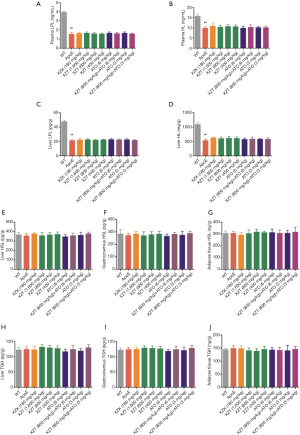
We first obtained plasma and liver from ApoE−/− mice and measured the expression of some sensitive markers of lipid metabolism-FFA, ox-LDL, visfatin, and adiponectin. As shown in Figure 3, compared with WT mice, high fat diet administration significantly increased the FFA and ox-LDL contents in plasma and liver. Concurrently, the expression level of plasma visfatin remarkably increased, and the expression level of plasma adiponectin significantly decreased (P<0.05), thereby indicating that mice treated with high-fat diet significantly inhibited lipid metabolism. However, high fat diet-induced lipid metabolism was attenuated by the drug treatment groups. For example, compared with ApoE−/− mice, ATO (6 and 3 mg/kg) administration for 34 weeks showed significant reduction in plasma visfatin, FFA, and ox-LDL contents in plasma and liver. However, the expression level of plasma adiponectin showed insignificant change (P>0.05). XZK administration for 34 weeks significantly lowered the plasma FFA and liver ox-LDL contents. Similar to the ATO group, XZT (1,600 mg/kg) administration for 34 weeks remarkably reduced the plasma visfatin, FFA, and ox-LDL contents in plasma and liver. However, the expression level of plasma adiponectin was significantly increased (P<0.05). XZT (800 mg/kg) administration for 34 weeks showed a remarkable decrease in plasma and liver ox-LDL contents but no significant effect on FFA, visfatin, and adiponectin. The ox-LDL content showed more significant decrease in mice cotreated with XZT (800 mg/kg) and ATO (6 mg/kg) than ATO (6 mg/kg)-treated mice.
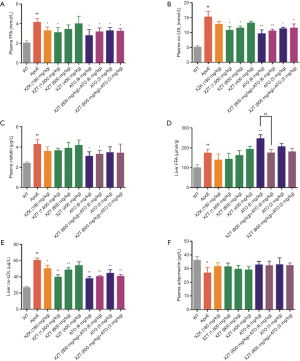
XZT attenuated high fat diet-induced FAS expression in ApoE−/− mice
As shown in Figure 4, the FAS (12) level in liver, skeletal muscle, and adipose tissue was upregulated in ApoE−/− mice compared with WT mice (P<0.01). Then, ATO (6 and 3 mg/kg) administration for 34 weeks significantly increased the FAS expression levels in liver and adipose tissues (P<0.01). This result indicates that atorvastatin increases the lipogenesis of liver and adipose tissue in ApoE−/− mice, which may lead to fatty liver. XZK administration for 34 weeks showed significant decrease in the FAS levels in the liver (P<0.01). XZT (1,600 and 800 mg/kg) administration for 34 weeks also remarkably decreased the FAS levels in liver and skeletal muscle (P<0.05). XZT (800 mg/kg) combined with ATO (6, 3 mg/kg) showed significant decrease in the liver FAS expression levels (P<0.05) compared with ATO (6 mg/kg) alone. This result suggests that XZT combined with ATO can inhibit the high fat diet-induced fatty liver in ApoE−/− mice.
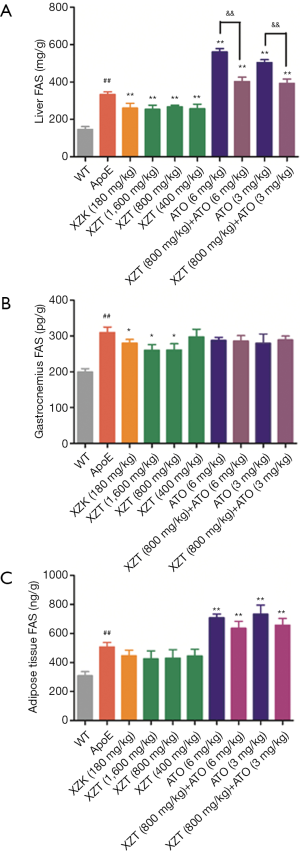
XZT increased the high fat diet-induced expression of LDLR in ApoE−/− mice
The upregulated HMG-CoAR and downregulated LDLR levels in the liver were observed in ApoE−/− mice compared with WT mice (P<0.01, Figure 5). Compared with ApoE−/− mice, the administration of ATO (6 and 3 mg/kg) for 34 weeks significantly decreased HMG-CoAR expression level (P<0.05) and increased the LDLR expression level in the liver (P<0.01). XZK administration for 34 weeks showed significant increase in LDLR expression level in the liver (P<0.01). XZT administration (1,600 and 800 mg/kg) for 34 weeks also showed remarkable increase in the liver LDLR expression level (P<0.05), thereby suggesting that the long-term administration of XZT can significantly upregulate the expression level of the cholesterol metabolism enzyme.
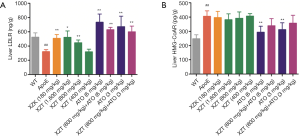
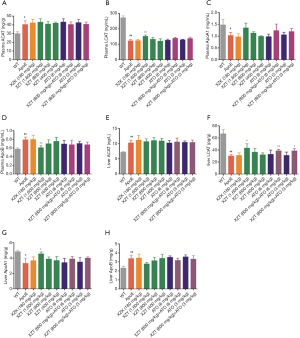
XZT increased high fat diet-induced expression of RCT in ApoE−/− mice
High fat diet administration decreased liver ABCA1 and SRB1 levels (Figure 6). However, ATO (6 and 3 mg/kg) and XZK administrations for 34 weeks substantially upregulated the liver SRB1 expression level (P<0.01) compared with ApoE−/− mice. XZT administration (1,600 and 800 mg/kg) for 34 weeks significantly upregulated the ABCA1 and SRB1 levels in the liver (P<0.05). Compared with ATO (6 and 3 mg/kg), the combination of XZT (800 mg/kg) and ATO (6 and 3 mg/kg) significantly increased the SRB1 level in the liver (P<0.05), thereby suggesting that XZT combined with ATO can promote cholesterol reverse transport in ApoE−/− mice induced by high fat diet.
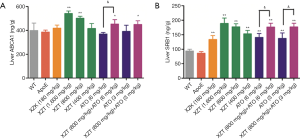
XZT increased high fat diet-induced expression of HDL in ApoE−/− mice
As shown in Figure 7, compared with WT mice, high fat diet administration significantly decreased the LCAT and ApoA1 levels in the plasma and liver, respectively (P<0.05). These changes were accompanied by an increase in the ACAT and ApoB levels (P<0.05). However, ATO (6 and 3 mg/kg) and XZK administrations exhibited insignificant effect on LCAT, ApoA1, ACAT, and ApoB expression levels in the plasma and liver for 34 weeks compared with ApoE−/− mice. XZT administration (1,600 mg/kg) for 34 weeks showed substantial increase in the LCAT and liver ApoA1 levels (P<0.05) and decrease in the plasma ApoB level (P<0.01). Compared with ATO (6 mg/kg), the combination of XZT (800 mg/kg) and ATO (6 mg/kg) remarkably upregulated the LCAT level in the plasma and liver (P<0.05), thereby indicating that XZT combined with ATO can significantly increase the HDL synthesis.
Discussion
Despite the development in treating patients with pathological hyperlipidemia, abnormal lipid homeostasis diseases exacerbate atherosclerotic heart diseases and remain a serious problem in cardiovascular studies. Considerable evidence has demonstrated that RCT and the accompanying increase in HDL levels play essential roles in hyperlipidemia development (13). Healing after high fat diet-induced hyperlipidemia provides a therapeutic possibility for attenuating hyperlipidemia. In this report, we described a potential potent therapeutic effect of XZT, with the main ingredient of Xie Bai, which can attenuate high fat diet-induced hyperlipidemia in atherosclerosis-prone mice. Considerable evidence indicates that XZT is closely correlated with RCT to develop HDL level and interfere with the subsequent catabolism of ApoA1.
Hyperlipidemia is a multifactorial clinical syndrome induced by a large number of physiological stimuli and pathological insults, including the excessive intakes of calories, saturated FAS, and cholesterol-rich fats (14). Several genetic factors, especially in the gene encoding enzymes characterized by the RCT system, are responsible in reversing hyperlipidemia incidence. However, negative environmental factors, such as alcoholism, lack of exercise, smoking, and high egg and fish intake, are closely related to the high hyperlipidemia incidence (15-18). In the present study, a generally recognized atherosclerosis model (3) involving ApoE−/− mice fed with high fat diet was applied to mimic the situation of patients with atherosclerotic heart disease suffering from hyperlipidemia in the clinic. Consistent with previous reports, ApoE−/− mice fed with high fat diet are susceptible to hyperlipidemia (3,19). However, XZT administration revealed remarkable advantages in alleviating the detrimental effects on abnormal lipid homeostasis disease in atherosclerosis-prone mice. To estimate the most effective XZT dose, we administered various clinical effective doses ranging from clinical equivalent dose (400 mg/kg) to fourfold clinical dose (1,600 mg/kg). Our data revealed that 800 and 1,600 mg/kg XZT exhibited remarkable effects on the levels of two typical hyperlipidemic markers, namely, LDL and HDL, in high fat diet-induced hyperlipidemia of ApoE−/− mice. Except for the sensitive serum lipid parameters, adipogenesis, cholesterol metabolism, and HDL synthesis accompanying the abnormal lipid homeostasis of failing hyperlipidemic disease were largely improved by XZT. To the best of our knowledge, this study is the first to establish the mechanism underlying the function of XZT in preventing hyperlipidemia development.
During hyperlipidemia development, we investigated the effect of XZT on the expression level of lipid metabolism enzymes in ApoE−/− mice. The lipogenic and lipolytic-related gene SREBP regulates the lipid balance in adipose tissue and liver, and induces the transcriptional expressions of FAS, HSL, TGH, HL, LPL, and other lipid and lipolysis-related genes (20,21). Among the genes, FAS promotes the conversion of adipose tissue into FAS by nutrient supply, and TG deposition also shows remarkable increase with the increase in FAS expression level (22). Therefore, the reduction in FAS activity can reduce fat synthesis. In the present study, high fat diet increased the lipogenesis in ApoE−/− mice, and these effects can be reversed by XZT for 34 weeks. Thus, the effects of XZT likely involve suppressing hyperlipidemia to blind the lipogenesis in the liver and gastrocnemius. The effects of high fat diet on fat accumulation were also substantially reversed by XZT (800 mg/kg) and ATO (6 and 3 mg/kg) incorporation in the liver and adipose tissue. High-fat diet can also upregulate FFA, ox-LDL, and visfatin activities in the plasma. This increase was remarkably suppressed by XZT treatment. Meanwhile, the capability of liver cells to absorb free cholesterol mainly depends on LDLR activity that exists in the membrane surface. LDLR expression was significantly upregulated, thereby promoting cholesterol endocytosis in the liver and reducing the serum cholesterol level (23,24). In the present study, the effects of high fat diet on the liver LDLR level was neutralized by XZT (1,600 and 800 mg/kg) administration.
HDL is involved in the development of hyperlipidemia through different mechanisms, such as vascular endothelial cell antiapoptosis and LDL-C anti-inflammation, and anti-oxidation (25). The RCT system regulated the HDL levels, which played important roles during the entire process (26). Cholesterol efflux is the first step in RCT, which plays a primary role in reducing lipids accumulation in the arterial wall and inhibiting the occurrence and development of hyperlipidemia. ABCA1 and SRB1 participated in the entire RCT process by mediating the cholesterol efflux from macrophages to ApoA1 and nascent HDL (27). The second step of RCT is to esterify free cholesterol in HDL to form cholesteryl ester by LCAT, which can be transferred directly to liver via SRBI or indirectly via CE transfer protein (CEPT) transfer to ApoB-containing lipoprotein and subsequently delivered to the liver via the LDL receptor (28). When ABCA1 and SRB1 expression was increased, the HDL level was improved. In the present study, we observed that the expression of ABCA1 and SRBI was upregulated in ApoE−/− mice treated with XZT. ACAT1 is a key enzyme used in intracellular cholesterol esterification and responsible in regulating the balance of intracellular free cholesterol and esterified cholesterol (29,30). LCAT catalyzes the conversion of extracellular neonatal cholesterol to cholesterol esters, thereby forming mature HDL (30). ApoA1 is also a major HDL apolipoprotein, which maintains the structural integrity of HDL, activates LCAT, and promotes plasma HDL to receive free cholesterol from peripheral tissues and converts it into cholesterol ester. ApoB is a main LDL and VLDL apolipoprotein, which can maintain the structural integrity of LDL and VLDL and participates in the LDL binding to atherosclerotic plaque (31). Our data also showed that LCAT, ApoA1, and ApoB release was remarkably changed by high fat diet treatment compared with WT mice. Meanwhile, XZT converted all the activities mentioned above due to high fat diet. Although the expression levels of the enzymes related to TG decreased after high fat diet administration, the downregulated LPL and HL expression levels were not increased by XZT treatment (Figure S2).
Although hypolipidemic drugs beneficially repress the cholesterol levels in clinical conditions, atherosclerotic CVDs worldwide, especially in Western countries, still present high mortality rates (32,33). Regardless of the availability of lipid-lowering drugs in lipid disorder therapy, considerable evidence significantly demonstrated that the elevated HDL levels caused by RCT are the common pathway for ameliorating unstable cholesterol accumulation. Clinical trials were used to detect the effective antihyperlipidemic drugs and HDL hypothesis. As the first class of drugs available, fibrates significantly reduced the plasma TC and slightly increased the HDL levels. Unfortunately, trials with fibrates showed that these drugs are weak and not beneficial for cardiovascular therapy (34,35). Another example is niacin, which is a lipid-lowering drug, that is used to treat hyperlipidemia by increasing the HDL, appropriately modifying lipid profiles, and inhibiting plaque formation in coronary arteries (36). However, a large trial by the National Institute of Health is criticized due to its small sample size and invalid experimental outcomes (37). Here, we demonstrated that XZT exhibited potent hyperlipidemia suppression and discussed its complex mechanisms. A comprehensive discussion on the similar pharmacological activities of several drugs indicated that the initially investigated drugs also possessed beneficial anticholesterol effects to optimize the treatment of atherosclerotic heart diseases, especially the synthesis of HDL (i.e., ACAT, LCAT, ApoA1, and ApoB). This finding is consistent with our current data.
In the present study, with the XZT concentration of <400 mg/kg, most indicators except liver FAS and SRB1 in atherosclerosis-prone mice cannot show significant change. This condition may be partly since 400 mg/kg XZT administration is ineffective in reducing hyperlipidemia in high fat diet-fed ApoE−/− mice. Different concentrations may result in insufficient distinct effects of the administration of XZT alone on lipid metabolism disorder. Despite the efficacy of hypolipidemic drugs, considerable evidence strongly indicated that the increase in HDL is an effective pathway for hyperlipidemia (38). This result may be the reason why atherosclerotic CVDs still present high mortality rates worldwide although lipid-lowering drugs are generally used in clinical settings.
In summary, the present study revealed that XZT treatment ameliorated lipid metabolism instability, induced RCT activation, and subsequently increased the HDL levels of high fat diet-fed ApoE−/− mice. The mechanisms by which XZT improved blood lipid dysfunction involved the preserved activation of RCT and the accompanying increase in the HDL level pathway. These findings clarified the mechanism underlying the function of XZT for the treatment of high fat diet-induced hyperlipidemia. If the therapeutic mechanisms of XZT are fully explored in animal models and patients, the XZT treatment can be an exceptional therapy for hospitalized patients with hyperlipidemia.
Acknowledgments
Funding: This study was supported by the Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (No. CAMS-I2M-1-010, CAMS-I2M-1-012).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Animal Care and Use Committee of Chinese Academy of Medical Sciences and Peking Union Medical College (SYXK 2017-0020).
References
- Hunter PM, Hegele RA. Functional foods and dietary supplements for the management of dyslipidaemia. Nat Rev Endocrinol 2017;13:278-88. [Crossref] [PubMed]
- Zhao Y, Chen ZY. Roles of Spicy Foods and Their Bioactive Compounds in Management of Hypercholesterolemia. J Agric Food Chem 2018;66:8662-71. [Crossref] [PubMed]
- Vasquez EC, Peotta VA, Gava AL, et al. Cardiac and vascular phenotypes in the apolipoprotein E-deficient mouse. J Biomed Sci 2012;19:22. [Crossref] [PubMed]
- Zhang L, Chen Y, Yang X, et al. MEK1/2 inhibitors activate macrophage ABCG1 expression and reverse cholesterol transport-An anti-atherogenic function of ERK1/2 inhibition. Biochim Biophys Acta 2016;1861:1180-91. [Crossref] [PubMed]
- Zhao ZW, Zhang M, Chen LY, et al. Heat shock protein 70 accelerates atherosclerosis by downregulating the expression of ABCA1 and ABCG1 through the JNK/Elk-1 pathway. Biochim Biophys Acta Mol Cell Biol Lipids 2018;1863:806-22. [Crossref] [PubMed]
- Kozarsky KF, Donahee MH, Rigotti A, et al. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature 1997;387:414-7. [Crossref] [PubMed]
- Istvan ES. Structural mechanism for statin inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Am Heart J 2002;144:S27-32. [Crossref] [PubMed]
- Lennernas H, Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences. Clin Pharmacokinet 1997;32:403-25. [Crossref] [PubMed]
- Li F, Xu Q, Zheng T, et al. Metabonomic analysis of Allium macrostemon Bunge as a treatment for acute myocardial ischemia in rats. J Pharm Biomed Anal 2014;88:225-34. [Crossref] [PubMed]
- Han C, Qi J, Gao S, et al. Vasodilation effect of volatile oil from Allium macrostemon Bunge are mediated by PKA/NO pathway and its constituent dimethyl disulfide in isolated rat pulmonary arterials. Fitoterapia 2017;120:52-7. [Crossref] [PubMed]
- Lee S, Kim DH, Lee CH, et al. Antidepressant-like activity of the aqueous extract of Allium macrostemon in mice. J Ethnopharmacol 2010;131:386-95. [Crossref] [PubMed]
- Hubner G, Brauchle M, Smola H, et al. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 1996;8:548-56. [Crossref] [PubMed]
- Hirata H, Uto-Kondo H, Ogura M, et al. Xanthohumol, a hop-derived prenylated flavonoid, promotes macrophage reverse cholesterol transport. J Nutr Biochem 2017;47:29-34. [Crossref] [PubMed]
- Ramachandran HD, Narasimhamurthy K, Raina PL. Modulation of cholesterol induced hypercholesterolemia through dietary factors in Indian desert gerbils (Merioneshurrianae). Nutr Res 2003;23:245-56. [Crossref]
- Lebold KM, Grant KA, Freeman WM, et al. Individual differences in hyperlipidemia and vitamin E status in response to chronic alcohol self-administration in cynomolgus monkeys. Alcohol Clin Exp Res 2011;35:474-83. [Crossref] [PubMed]
- Bragg DA, Walling A. Metabolic Syndrome: Hyperlipidemia. FP Essent 2015;435:17-23. [PubMed]
- Magan-Fernandez A, Papay-Ramirez L, Tomas J, et al. Association of simvastatin and hyperlipidemia with periodontal status and bone metabolism markers. J Periodontol 2014;85:1408-15. [Crossref] [PubMed]
- Weggemans RM, Zock PL, Katan MB. Dietary cholesterol from eggs increases the ratio of total cholesterol to high-density lipoprotein cholesterol in humans: a meta-analysis. Am J Clin Nutr 2001;73:885-91. [Crossref] [PubMed]
- Wu JH, Hagaman J, Kim S, et al. Aortic constriction exacerbates atherosclerosis and induces cardiac dysfunction in mice lacking apolipoprotein E. Arterioscler Thromb Vasc Biol 2002;22:469-75. [Crossref] [PubMed]
- Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res 2007;68:72-82. [PubMed]
- Tang JJ, Li JG, Qi W, et al. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab 2011;13:44-56. [Crossref] [PubMed]
- Liu C, Shen YJ, Tu QB, et al. Pedunculoside, a novel triterpene saponin extracted from Ilex rotunda, ameliorates high-fat diet induced hyperlipidemia in rats. Biomed Pharmacother 2018;101:608-16. [Crossref] [PubMed]
- Minicocci I, Prisco C, Montali A, et al. Contribution of mutations in low density lipoprotein receptor (LDLR) and lipoprotein lipase (LPL) genes to familial combined hyperlipidemia (FCHL): a reappraisal by using a resequencing approach. Atherosclerosis 2015;242:618-24. [Crossref] [PubMed]
- O'Hare EA, Wang X, Montasser ME, et al. Disruption of ldlr causes increased LDL-c and vascular lipid accumulation in a zebrafish model of hypercholesterolemia. J Lipid Res 2014;55:2242-53. [Crossref] [PubMed]
- Nakamura A, Niimura H, Kuwabara K, et al. Gene-gene combination effect and interactions among ABCA1, APOA1, SR-B1, and CETP polymorphisms for serum high-density lipoprotein-cholesterol in the Japanese population. PLoS One 2013;8:e82046. [Crossref] [PubMed]
- Tsompanidi EM, Brinkmeier MS, Fotiadou EH, et al. HDL biogenesis and functions: role of HDL quality and quantity in atherosclerosis. Atherosclerosis 2010;208:3-9. [Crossref] [PubMed]
- Tang SL, Zhao ZW, Liu SM, et al. Pregnancy-Associated Plasma Protein-A Accelerates Atherosclerosis by Regulating Reverse Cholesterol Transport and Inflammation. Circ J 2019;83:515-23. [Crossref] [PubMed]
- Rader DJ, Alexander ET, Weibel GL, et al. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res 2009;50 Suppl:S189-94. [Crossref] [PubMed]
- Fujiwara Y, Kiyota N, Tsurushima K, et al. Tomatidine, a tomato sapogenol, ameliorates hyperlipidemia and atherosclerosis in ApoE-deficient mice by inhibiting acyl-CoA: cholesterol acyl-transferase (ACAT). J Agric Food Chem 2012;60:2472-9. [Crossref] [PubMed]
- Vaziri ND, Liang K. ACAT inhibition reverses LCAT deficiency and improves plasma HDL in chronic renal failure. Am J Physiol Renal Physiol 2004;287:F1038-43. [Crossref] [PubMed]
- Junyent M, Zambon D, Gilabert R, et al. Carotid atherosclerosis in familial combined hyperlipidemia associated with the APOB/APOA-I ratio. Atherosclerosis 2008;197:740-6. [Crossref] [PubMed]
- Charo IF. Blinding the monocytes to protect the heart. Circulation 2013;127:2006-8. [Crossref] [PubMed]
- Koh KK, Han SH, Oh PC, et al. Combination therapy for treatment or prevention of atherosclerosis: focus on the lipid-RAAS interaction. Atherosclerosis 2010;209:307-13. [Crossref] [PubMed]
- Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563-74. [Crossref] [PubMed]
- Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366:1849-61. [Crossref] [PubMed]
- Villines TC, Stanek EJ, Devine PJ, et al. The ARBITER 6-HALTS Trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis): final results and the impact of medication adherence, dose, and treatment duration. J Am Coll Cardiol 2010;55:2721-6. [Crossref] [PubMed]
- Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255-67. [Crossref] [PubMed]
- Fless GM, Juhn D, Karlin J, et al. Response of rhesus serum high density lipoproteins to cycles of diet-induced hypercholesterolemia. Arteriosclerosis 1984;4:154-64. [Crossref] [PubMed]


