Diagnosis of acute respiratory distress syndrome by exhaled breath analysis
Acute respiratory distress syndrome (ARDS)
The ARDS is a complication of critical illness that is characterized by acute onset, protein rich, pulmonary edema (1). The clinical diagnosis is established based on the radiological, physiological and clinical criteria summarized in the Berlin definition (2): (I) acute onset of hypoxemia characterized as a PaO2/FiO2 below 300 mmHg; (II) bilateral opacities on chest radiography consistent with pulmonary edema; and (III) cardiac dysfunction is insufficient explanation for the previous findings. ARDS is a frequently encountered problem on intensive care units, with about 10% of the patients fulfilling the criteria (3). Hospital mortality remains about 40%, despite improvements in ventilatory support (3).
The management of patients with ARDS currently includes all measurements taken for the general critically ill ICU population in combination with adjustments of the ventilator settings to reduce additional lung injury via mechanical ventilation (4). In patients with more severe hypoxemia ventilator induced lung injury can be further reduced through prone positioning or neuromuscular blockade (5,6). None of the many pharmacological interventions that have been tested have shown any benefit in survival for those treated with the drug up to now (7). The non-superiority of these interventions may be explained by two facts inherent to the diagnostic criteria for ARDS. First, ARDS is a biologically heterogeneous syndrome. This is supported by the plethora of pathological diagnoses that can be made in post-mortem studies of the lung of patients with ARDS (8,9). Furthermore, biological data from living ARDS patients suggest that there are least two subtypes of patients, with different biological profiles and clinical characteristics (10-12). Second, ARDS is only diagnosed when the lungs are already flooded with edema. This is fundamental to the use of hypoxemia and chest radiography as diagnostic criteria. Pharmacological interventions frequently aim to limit the formation of pulmonary edema, but might be less effective when edema has already formed. Thus, while the definition is very well equipped for the epidemiological description of the syndrome it might not be suited for the selection of patients that might benefit most from specific treatments.
In this review, we will review the possibility of using breath analysis as an early diagnostic tool for ARDS. There is still limited data on the diagnostic accuracy of breath tests, but we will provide a rationale on why the technique required further investigation.
Prediction and/or early diagnosis of ARDS
Prediction and early diagnosis both involve the quantification of the risk of an individual patient on the development of ARDS, before the patient fulfills the clinical criteria. The main difference between prediction and early diagnosis is that the former aims to assess the probability of ARDS in patients who do not show any signs of the disease, while the latter identifies pathophysiological phenomena that facilitate an earlier diagnosis.
The lung injury prediction score (LIPS) has been the best effort up to now to construct a prediction tool for ARDS based on clinical characteristics (13). It performed well in terms of discrimination in an internal and external validation cohort and reached an area under the receiver operating characteristics curve of around 0.80 (13). However, in several other studies where the tool was used to select patients at risk for ARDS the predicted probability did not correspond to the observed probability, with a much lower prevalence than expected (14,15). The combined results show that prediction based on clinical variables alone might be insufficient and that it might need to be combined with early diagnosis (and thus detection of pathophysiological changes).
Biomarkers in plasma might be suitable candidates as markers for early diagnosis of ARDS as plasma is easily obtained and can be analyzed quickly. Angiopoietin-2 was found to be higher in patients in the emergency room who fulfilled the criteria for ARDS in subsequent days than in those who did not (16). More importantly, it added to the diagnostic accuracy of LIPS with a combined area under the ROC curve of 0.84. However, promising the results of this study, the study population was small and the findings are not yet reproduced independently, so the diagnostic accuracy of angiopoietin-2 requires careful consideration and it cannot be implemented into clinical practice at this moment.
Broncho-alveolar lavage (BAL) fluid might contain more information on the pathophysiological processes leading to ARDS than plasma. Indeed, landmark studies from the previous century suggest that inflammation is limited to the lung in the early phases of ARDS and that compartmentalization is only lost after severe pulmonary damage has occurred (17,18). However, the logistics of obtaining BAL fluid, or any other alveolar sample for that matter, are too difficult to use as an early diagnostic tool in patients at high risk for ARDS.
Exhaled breath analysis
The exchange of oxygen and carbon dioxide is the primary function of the lung. These molecules, together with nitrogen and water, attribute for almost all the volume in breath. However, upon deeper inspection breath contains hundreds of lower abundance compounds present in the range of part-per-trillion to part-per-million. Many of these molecules are volatile organic compounds (VOCs); organic molecules produced through the human metabolism or by the microbiome (19), others are inhaled from the environment or degradation products of medications or toxins. VOCs in the exhaled breath have been linked to glucose metabolism (20), cholesterol metabolism (21), lipid peroxidation (22), bacterial growth (23,24) and malignant cell growth (25).
The major advantage of exhaled breath analysis over analysis of biomarkers in fluids are: (I) the material is easily obtained; (II) the sampling procedure can be repeated endlessly; (III) the detection method can be performed bedside after the exact biomarker is known or when only the pattern of molecules is studied and not the individual molecules; and (IV) the results are available (almost) without delay. As we explore these advantages, several reservations should be made on their application, which also highlight the disadvantages of breath analysis.
Exhaled breath is easy to obtain as we exhale about two thirds of the time. However, as recently has been summarized in a technical standard from the European Respiratory Society, breath needs to be collected using the same protocols with regard to flow, volume, resistance and sampling materials in each patient (26). This results in practical problems as patients with shortness of breath cannot exhale the same way as healthy volunteers or even hospitalized patients without pulmonary symptoms do. Therefore, the sampling method should be optimized for the target population and not for healthy subjects.
The sampling procedure can be repeated frequently, but each time breath has to be analyzed on-line or it has to be stored (19). Storage can be done for a short period of time in special bags, which limit the contamination of the sample (27). For longer storage, the VOCs have to be loaded onto a storage tube filled with an absorbent material such as Tenax (28,29). Another advantage of storage on an absorption tube is that the sample can be concentrated by, for example desorbing a volume of 1L of VOCs from exhaled breath into 10 mL of nitrogen gas. The major disadvantage of storage is that the molecular composition of the mixture will change and that it might not represent the clinical situation anymore, where an answer is required in minutes rather than days. For biomarker discovery studies, storage and transport of a sample to an analytical chemistry facility is typically necessary, but this further complicates the direct application of results into clinical practice.
The detection of a VOC is possible at the bedside, but only after the target VOC is known. Untargeted analysis of exhaled breath requires metabolomics platforms such as gas-chromatography and mass-spectrometry (GC-MS); laborious machines that require expertise for operation. When discovery studies provide one or several target molecules other analytical methods can be used to detect those at the bedside [such as compact GC, laser spectroscopy based methods or selective biochemical sensors; for a review on the techniques see reference (19,26)]. An alternative method for the detection of profiles of VOC is electronic nose (eNose) (30). eNoses do not separate, identify or quantify individual VOCs, but rather respond to the mixture of gasses. It received its name after the similarities with mammalian smell; we don’t smell one molecule but recognize mixtures and couple those patterns to conclusions. The eNose works in a similar way; it is trained to recognize patterns of “smells” and couples those do specific disease states. If new mixtures are presented to the eNose, it will compare those to the already learned patterns and provide a probability of diagnosis. eNoses have been successfully trained in the recognition of several pulmonary disease states (31-33).
In theory, the results of a breath test can be readily available at the bedside. However, the current technological readiness level makes the analysis of each sample cumbersome and very time consuming as GC-MS analysis requires extensive calibration and the statistical analysis is highly sophisticated. A bedside diagnostic result can only be provided when the three previous steps are harmonized and have let to acceptable results (Figure 1).
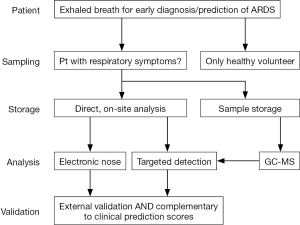
Breath sampling in intubated and mechanically ventilated patients
Multiple sampling methods have been used for breath collection in the ICU, ranging from a manually operated switch on the expiratory valve (34,35), automated CO2 regulated alveolar sampling (36), continuous breath collection with a side stream connector close to the endotracheal tube (37) and through an endotracheal suction catheter (38). These methods have not been compared head-to-head for the diagnosis of ARDS. Based on physiological arguments, it is reasonable to prefer CO2 based methods for sampling alveolar air for the detection of blood-borne VOCs (19,26). For ARDS however, it is not established if this provides more information on top of analyses of the whole exhaled breath (including the dead space portion).
Exhaled breath metabolites of interest
Isoprene
Isoprene was proposed as an exhaled breath marker for ARDS in 1998 (34). It was hypothesized that isoprene was produced through cholesterol metabolism and experimental data showed that these pathways were also altered in patients with ARDS (34,39). However, isoprene has never been validated as a diagnostic marker afterwards and a recent study could not replicate the results (40). Other groups have suggested that isoprene originates from muscle cells rather than cholesterol metabolism under most circumstances and this may be a huge confounder (41). It may be speculated that isoprene was a marker when ARDS patients were still regularly paralyzed and the contribution of muscle activity was minimal, but that this signal was diluted with 21st century sedation protocols and decreased use of muscle paralysis.
Alkanes
Alkanes in exhaled breath are known to originate from lipid peroxidation (42). Ethane, the smallest alkane, was identified as a marker of oxidative stress more than 50 years ago (43). Since, we have learned that peroxidation of different fatty acids results in different profile of alkanes (42). Octane was shown to be predictive of ARDS in a cohort of ventilated ICU patients and this result was validated in a temporal external validation cohort (40). Only the peroxidation of oleic acid results in the production octane (42,44). Observational studies have shown that oleic acid and lipid peroxidation are increased in plasma of patients with ARDS (45), which might explain why only octane is increased rather than all alkanes. Furthermore, oleic acid is a well-known compound to experimentally induce ARDS in animals and has profound effects on oxidative stress itself (46-48).
Acetaldehyde
Acetaldehyde was identified as a marker of ARDS in the same study that provided octane as a marker (40). Acetaldehyde has been linked to bacterial metabolism in vitro (23), but airway colonization was not a confounding factor. Several recent studies suggest the enrichment of Enterobacteriaceae in the lungs of ARDS patients and it could be speculated that these communities contribute to the exhaled concentration of acetaldehyde (49,50). Alternatively, acetaldehyde can be produced by leukocytes (51), but little is known about the VOCs produced by, for example, activated neutrophils.
Ethylene
Ethylene is a well-established marker of lipid peroxidation and it’s production is independent of the type of unsaturated fatty acid that is oxidized (52). Ethylene was not directly linked to ARDS development in clinical studies but was increased during events known to cause oxidative stress during cardiac surgery and systematic inflammatory response syndrome (53,54). Interestingly, ethylene is a very early marker of oxidative stress and seems to increase almost instantaneously, making it more suitable for monitoring purposes.
Other
Exhaled breath was studied in several animal models that can be regarded surrogates for acute lung injury (55). LPS and mechanical ventilation were used as “hits” to provoke acute lung injury. A large range of changes was found in the breath of the injured rats, when compared to control animals (56,57). Most of these molecules can be linked to changes in metabolism of amino acids or oxidative stress. However, the exact molecules do not match those found in human experiments. It can be hypothesized that rodents have, for example, a different composition of fatty acids in their lungs and therefore produce other lipid peroxidation products, but this theory is highly speculative. The discordant findings might also just represent the difference between ARDS in humans and experimentally induced lung injury in rodents (55).
Contaminants of breath
Anesthetics
Anesthetics such as propofol, isoprene and sevoflurane are detectable in the exhaled breath of patients who have been exposed to these molecules. Propofol is administrated intravenously and passes the blood-air barrier in the lungs. The concentration of propofol in breath is proportional to the blood concentration (58-60). The concentrations are quite low (parts-per-billion) and do not disturb the rest of the exhaled breath signal. Inhaled anesthetics on the other hand can be omnipresent in exhaled breath, even when not administrated anymore at the moment of sampling. Sevoflurane has been found in the breath of health care professionals working in the operating rooms (61). Even days after surgery, sevoflurane can still be found in high parts-per-billion concentration. Furthermore, as it is very volatile it typically presents at the beginning of the chromatogram and may shift retention times of many compounds with similar chromatographic properties (personal communication). The inability of eNoses to separate the anesthetic signals from disease specific signals might explain why the diagnostic accuracy of these machines is so much lower in critically ill patients than in the spontaneously breath individuals (62).
Tubing
The ventilator circuit contains many types of plastics all of which may release volatiles that might disturb the exhaled breath signal. A thorough check of the background signal is needed before exhaled breath analysis can be set up (37). The endotracheal tube also contributed to the collection of exhaled VOCs, most notably through the addition of cyclohexanone (63).
Towards a clinical application of a breath test
Several steps have to be taken before any breath test can be used for the diagnosis and/or prediction of ARDS. First, all the markers require further validation in a truly independent multi-center cohort. Such a cohort should be of sufficient size to allow for the quantification of confounding and moderating effects of ventilator settings, comorbidities and colonization of the respiratory tract. The exhaled breath test should also be compared to other techniques for ARDS prediction, such as plasma biomarkers and clinical scores like the LIPS (64). The additive value of a breath test to the LIPS should also be evaluated.
Second, many of the detection methods are too laborious at the moment. This means that the machine required for detection of the molecule are too expensive, require specialized personal, have considerable down-time and results take too long for an intensive care setting. Special attention should be given towards the development of an on-site analytical device. Ideally, the machine could be operated by a nurse and provide rapid results—very similar to the decentralized blood gas analyzers that are commonly used nowadays. A second possibility is the integration of the breath analyzer into the ventilator, providing continuous results. Continuous exhaled breath monitoring in mechanically ventilated patients has been tried with an eNose, but gave very difficult to interpret results (65,66). Also, the technological challenges are more profound when the breath analyzer has to integrated into the ventilator circuit and the costs will increase due to the necessity of one breath analyzed per patient. It would also require the ventilator company to acquire the technology for continuous breath analysis and the technology would only be available for those patients who are ventilated with the machine from that particular company. It seems that a decentralized lab option is more feasible in the near future than the integration of a breath analyzer into the ventilator circuit, although this might be possible on the longer term.
Conclusions
The currently available evidence suggests that exhaled breath contains several molecules that may serve as diagnostic or predictive biomarkers for ARDS. Most of the candidate markers can be linked to lipid peroxidation. Only octane has been validated in a temporal external validation cohort and is, at this moment, the top-ranking breath biomarker for ARDS. Thorough independent validation has to be performed for all identified potential biomarkers and the breath tests have to evolve to qualify for clinical use. A decentralized breath analyzer that can be operated by nurses is probably the most feasible scenario in the near future.
Acknowledgements
LD Bos is supported by a personal research grant from the Dutch lung foundation (longfonds), he is supported through the FP-7 IAPP grant supporting the breathed consortium and he is supported through Health Holland and the Dutch lung foundation (longfonds) through the private-public partnership (PPP) grant for the DARTS consortium.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med 2017;377:562-72. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: The berlin definition. JAMA 2012;307:2526-33. [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Rittayamai N, Brochard L. Recent advances in mechanical ventilation in patients with acute respiratory distress syndrome. Eur Respir Rev 2015;24:132-40. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Boyle AJ, Mac Sweeney R, McAuley DF. Pharmacological treatments in ARDS; a state-of-the-art update. BMC Med 2013;11:166. [Crossref] [PubMed]
- Esteban A, Fernández-Segoviano P, Frutos-Vivar F, et al. Comparison of clinical criteria for the acute respiratory distress syndrome with autopsy findings. Ann Intern Med 2004;141:440-5. [Crossref] [PubMed]
- Thille AW, Esteban A, Fernández-Segoviano P, et al. Comparison of the Berlin Definition for Acute Respiratory Distress Syndrome with Autopsy. Am J Respir Crit Care Med 2013;187:761-7. [Crossref] [PubMed]
- Famous KR, Delucchi K, Ware LB, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med 2017;195:331-8. [PubMed]
- Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014;2:611-20. [Crossref] [PubMed]
- Bos LD, Schouten LR, van Vught LA, et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax 2017;72:876-83. [Crossref] [PubMed]
- Trillo-Alvarez C, Cartin-Ceba R, Kor DJ, et al. Acute lung injury prediction score: derivation and validation in a population-based sample. Eur Respir J 2011;37:604-9. [Crossref] [PubMed]
- Neto AS, Barbas CSV, Simonis FD, et al. Epidemiological characteristics, practice of ventilation, and clinical outcome in patients at risk of acute respiratory distress syndrome in intensive care units from 16 countries (PRoVENT): an international, multicentre, prospective study. Lancet Respir Med 2016;4:882-93. [Crossref] [PubMed]
- Kor DJ, Carter RE, Park PK, et al. Effect of Aspirin on Development of ARDS in At-Risk Patients Presenting to the Emergency Department: The LIPS-A Randomized Clinical Trial. JAMA 2016;315:2406-14. [Crossref] [PubMed]
- Agrawal A, Matthay MA, Kangelaris KN, et al. Plasma Angiopoietin-2 Predicts the Onset of Acute Lung Injury in Critically Ill Patients. Am J Respir Crit Care Med 2013;187:736-42. [Crossref] [PubMed]
- Pugin J, Verghese G, Widmer MC, et al. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med 1999;27:304-12. [Crossref] [PubMed]
- Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;164:1896-903. [Crossref] [PubMed]
- Boots AW, Bos LD, van der Schee MP, et al. Exhaled Molecular Fingerprinting in Diagnosis and Monitoring: Validating Volatile Promises. Trends Mol Med 2015;21:633-44. [Crossref] [PubMed]
- Leopold JH, van Hooijdonk RT, Sterk PJ, et al. Glucose prediction by analysis of exhaled metabolites – a systematic review. BMC Anesthesiol 2014;14:46. [Crossref] [PubMed]
- Scholpp J, Schubert JK, Miekisch W, et al. Breath markers and soluble lipid peroxidation markers in critically ill patients. Clin Chem Lab Med 2002;40:587-94. [Crossref] [PubMed]
- Van Gossum A, Decuyper J. Breath alkanes as an index of lipid peroxidation. Eur Respir J 1989;2:787-91. [PubMed]
- Bos LDJ, Sterk PJ, Schultz MJ. Volatile Metabolites of Pathogens: A Systematic Review. PLoS Pathog 2013;9:e1003311. [Crossref] [PubMed]
- Bos LD, Meinardi S, Blake D, et al. Bacteria in the airways of patients with cystic fibrosis are genetically capable of producing VOCs in breath. J Breath Res 2016;10:047103. [Crossref] [PubMed]
- Rutter AV, Chippendale TW, Yang Y, et al. Quantification by SIFT-MS of acetaldehyde released by lung cells in a 3D model. Analyst 2013;138:91-5. [Crossref] [PubMed]
- Horváth I, Barnes PJ, Loukides S, et al. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J 2017;49:1600965. [Crossref] [PubMed]
- Mochalski P, King J, Unterkofler K, et al. Stability of selected volatile breath constituents in Tedlar, Kynar and Flexfilm sampling bags. Analyst 2013;138:1405-18. [Crossref] [PubMed]
- Harshman SW, Mani N, Geier BA, et al. Storage stability of exhaled breath on Tenax TA. J Breath Res 2016;10:046008. [Crossref] [PubMed]
- van der Schee MP, Fens N, Brinkman P, et al. Effect of transportation and storage using sorbent tubes of exhaled breath samples on diagnostic accuracy of electronic nose analysis. J Breath Res 2013;7:016002. [Crossref] [PubMed]
- Wilson AD, Baietto M. Applications and advances in electronic-nose technologies. Sensors (Basel) 2009;9:5099-148. [Crossref] [PubMed]
- de Vries R, Brinkman P, van der Schee MP, et al. Integration of electronic nose technology with spirometry: validation of a new approach for exhaled breath analysis. J Breath Res 2015;9:046001. [Crossref] [PubMed]
- Brinkman P, van de Pol MA, Gerritsen MG, et al. Exhaled breath profiles in the monitoring of loss of control and clinical recovery in asthma. Clin Exp Allergy 2017;47:1159-69. [Crossref] [PubMed]
- Fens N, de Nijs SB, Peters S, et al. Exhaled air molecular profiling in relation to inflammatory subtype and activity in COPD. Eur Respir J 2011;38:1301-9. [Crossref] [PubMed]
- Schubert JK, Muller WP, Benzing A, et al. Application of a new method for analysis of exhaled gas in critically ill patients. Intensive Care Med 1998;24:415-21. [Crossref] [PubMed]
- Birken T, Schubert J, Miekisch W, et al. A novel visually CO2 controlled alveolar breath sampling technique. Technol Health Care 2006;14:499-506. [PubMed]
- Miekisch W, Hengstenberg A, Kischkel S, et al. Construction and Evaluation of a Versatile CO2 Controlled Breath Collection Device. IEEE Sens J 2010;10:211-5. [Crossref]
- Bos LD, Wang Y, Weda H, et al. A simple breath sampling method in intubated and mechanically ventilated critically ill patients. Respir Physiol Neurobiol 2014;191:67-74. [Crossref] [PubMed]
- Fowler SJ, Xu Y, Basanta M, et al. Breath Analysis On The Intensive Care Unit: Screening Invasively Ventilated Patients For Lower Respiratory Tract Pathogens. Am J Respir Crit Care Med 2013;187:A1322.
- Feingold KR, Hardardottir I, Memon R, et al. Effect of endotoxin on cholesterol biosynthesis and distribution in serum lipoproteins in Syrian hamsters. J Lipid Res 1993;34:2147-58. [PubMed]
- Bos LD, Weda H, Wang Y, et al. Exhaled breath metabolomics as a noninvasive diagnostic tool for acute respiratory distress syndrome. Eur Respir J 2014;44:188-97. [Crossref] [PubMed]
- King J, Mochalski P, Unterkofler K, et al. Breath isoprene: Muscle dystrophy patients support the concept of a pool of isoprene in the periphery of the human body. Biochem Biophys Res Commun 2012;423:526-30. [Crossref] [PubMed]
- Kneepkens CM, Ferreira C, Lepage G, et al. The hydrocarbon breath test in the study of lipid peroxidation: principles and practice. Clin Invest Med 1992;15:163-86. [PubMed]
- Riely CA, Cohen G, Lieberman M. Ethane evolution: a new index of lipid peroxidation. Science 1974;183:208-10. [Crossref] [PubMed]
- Kneepkens CM, Lepage G, Roy CC. The potential of the hydrocarbon breath test as a measure of lipid peroxidation. Free Radic Biol Med 1994;17:127-60. [Crossref] [PubMed]
- Quinlan GJ, Lamb NJ, Evans TW, et al. Plasma fatty acid changes and increased lipid peroxidation in patients with adult respiratory distress syndrome. Crit Care Med 1996;24:241-6. [Crossref] [PubMed]
- Carrillo C, Cavia Mdel M, Alonso-Torre S. Role of oleic acid in immune system; mechanism of action; a review. Nutr Hosp 2012;27:978-90. [PubMed]
- Yang C, Moriuchi H, Takase J, et al. Oxidative stress in early stage of acute lung injury induced with oleic acid in guinea pigs. Biol Pharm Bull 2003;26:424-8. [Crossref] [PubMed]
- Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L379-99. [Crossref] [PubMed]
- Dickson RP, Singer BH, Newstead MW, et al. Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol 2016;1:16113. [Crossref] [PubMed]
- Panzer AR, Lynch SV, Langelier C, et al. Lung Microbiota is Related to Smoking Status and to Development of ARDS in Critically Ill Trauma Patients. Am J Respir Crit Care Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Shin HW, Umber BJ, Meinardi S, et al. Acetaldehyde and hexanaldehyde from cultured white cells. J Transl Med 2009;7:31. [Crossref] [PubMed]
- Bousquet JF, Thimann KV. Lipid peroxidation forms ethylene from 1-aminocyclopropane-1-carboxylic acid and may operate in leaf senescence. Proc Natl Acad Sci 1984;81:1724-7. [Crossref] [PubMed]
- Cristescu SM, Kiss R, Hekkert St, et al. Real-time monitoring of endogenous lipid peroxidation by exhaled ethylene in patients undergoing cardiac surgery. Am J Physiol Lung Cell Mol Physiol 2014;307:L509-15. [Crossref] [PubMed]
- Paardekooper LM, van den Bogaart G, Kox M, et al. Ethylene, an early marker of systemic inflammation in humans. Sci Rep 2017;7:6889. [Crossref] [PubMed]
- Matute-Bello G, Downey G, Moore BB, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 2011;44:725-38. [Crossref] [PubMed]
- Guaman AV, Carreras A, Calvo D, et al. Rapid detection of sepsis in rats through volatile organic compounds in breath. J Chromatogr B Analyt Technol Biomed Life Sci 2012;881-882:76-82. [Crossref] [PubMed]
- Bos LD, van Walree IC, Kolk AH, et al. Alterations of Exhaled Breath Metabolite-mixtures in Two Rat Models of Lipopolysaccharide-induced Lung Injury. J Appl Physiol 2013;115:1487-95. [Crossref] [PubMed]
- Li X, Martinez-Lozano Sinues P, Dallmann R, et al. Drug Pharmacokinetics Determined by Real-Time Analysis of Mouse Breath. Angew Chem Int Ed Engl 2015;54:7815-8. [Crossref] [PubMed]
- Takita A, Masui K, Kazama T. On-line monitoring of end-tidal propofol concentration in anesthetized patients. Anesthesiology 2007;106:659-64. [Crossref] [PubMed]
- Chen X, Zhang XL, Liu L, et al. Gas chromatograph-surface acoustic wave for quick real-time assessment of blood/exhaled gas ratio of propofol in humans. Br J Anaesth 2014;113:807-14. [Crossref] [PubMed]
- Trefz P, Schmidt M, Oertel P, et al. Continuous real time breath gas monitoring in the clinical environment by proton-transfer-reaction-time-of-flight-mass spectrometry. Anal Chem 2013;85:10321-9. [Crossref] [PubMed]
- Bos LD, Schultz MJ, Sterk PJ. Exhaled breath profiling for diagnosing acute respiratory distress syndrome. BMC Pulm Med 2014;14:72. [Crossref] [PubMed]
- Kischkel S, Miekisch W, Fuchs P, et al. Breath analysis during one-lung ventilation in cancer patients. Eur Respir J 2012;40:706-13. [Crossref] [PubMed]
- Gajic O, Dabbagh O, Park PK, et al. Early Identification of Patients at Risk of Acute Lung Injury: Evaluation of Lung Injury Prediction Score in a Multicenter Cohort Study. Am J Respir Crit Care Med 2011;183:462-70. [Crossref] [PubMed]
- Leopold JH, Abu-Hanna A, Colombo C, et al. Factors influencing continuous breath signal in intubated and mechanically-ventilated intensive care unit patients measured by an electronic nose. Sensors (Basel) 2016;16:E1337. [Crossref] [PubMed]
- Leopold JH, Bos LD, Colombo C, et al. Non-invasive breath monitoring with eNose does not improve glucose diagnostics in critically ill patients in comparison to continuous glucose monitoring in blood. J Breath Res 2017;11:026002. [Crossref] [PubMed]


