Correlation between electrocardiographic changes and coronary findings in patients with acute myocardial infarction and single-vessel disease
Introduction
The electrocardiogram (ECG) is essential in the evaluation of patients with suspected acute myocardial infarction (AMI). Correlation of ST-segment elevation on the 12-lead ECG with the expected affected coronary territory is well established in patients with ST-elevation myocardial infarction (STEMI) (1). Non-ST-elevation myocardial infarction (NSTEMI) usually affects older patients with multiple comorbidities and multi-vessel coronary artery disease (CAD). In patients with NSTEMI, correlation of ischemic ECG abnormalities with the affected coronary territory has not been well-established (2). We assessed the correlation between ischemic ECG changes and the affected coronary territory in patients with STEMI and NSTEMI and 1-vessel CAD.
Methods
We conducted a retrospective review of the medical records of all patients referred for coronary angiography at our institution in the year 2012. We selected patients with obstructive CAD in a single coronary territory [left anterior descending artery (LAD), right coronary artery (RCA), or left circumflex artery (LCX)]. Obstructive 1-vessel CAD was defined as greater than 50% occlusion of a main coronary artery or important branch vessel, with less than 50% occlusion of all other coronary arteries. From this group, we then selected those patients who presented with AMI, defined as at least one elevated troponin I level (>0.04 ng/mL as defined by the local laboratory) and presenting symptoms of chest pain, acute dyspnea, or both.
All ECGs obtained during the index hospitalization, as well as previous ECGs (when available for comparison) were interpreted by a single experienced electrocardiography (WSA) who was blinded to patient history, angiographic findings, and previous ECG interpretation. The ECG criteria for STEMI were new ST elevation at the J point in two contiguous leads with the cut-points of ≥0.1 mV in all leads other than leads V1–V3, where elevation ≥0.2 mV was required. In the absence of criteria for STEMI, definite ischemic abnormalities were considered to be present if there was new horizontal or down-sloping ST depression ≥0.05 mV in two contiguous leads or T-wave inversion ≥0.1 mV in two contiguous leads with prominent R wave or R/S ratio >1 or both (3). The ECG interpretations were reported as (I) no definite ischemic changes; (II) infarction if STEMI criteria were met; (III) definite ischemic ECG changes in the absence of criteria for STEMI, either anterior (V1–V4), inferior (II, III, aVF), or lateral (I, aVL, V5, V6) (4).
Results
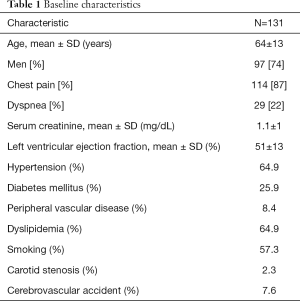
We identified 131 consecutive patients with AMI and 1-vessel CAD. These included 97 men (74%) and 34 women (26%), with a mean age 64±13 years (Table 1). According to blinded ECG review, 29 (22%) had STEMI and 102 (78%) had NSTEMI (Table 2). Among 11 patients with anterior STEMI, all 11 (100%) had LAD obstructive CAD, whereas among 18 patients with inferior STEMI, 14 (78%) had RCA obstructive CAD, 3 (17%) had LCX obstructive CAD, and 1 (5%) had LAD obstructive CAD. Of 102 patients with NSTEMI, 53 patients (52%) had definite ischemic ECG abnormalities. Among these, 31 (59%) had anterior ECG ischemic abnormalities, and 22 had inferior ECG ischemic abnormalities. Of the 31 NSTEMI patients with anterior ECG ischemic abnormalities, 30 (97%) had LAD obstructive CAD, and 1 (3%) had RCA obstructive CAD. Of the 22 NSTEMI patients with inferior ECG ischemic abnormalities, 14 (64%) had RCA obstructive CAD, 5 (23%) had LCX obstructive CAD, and 3 (14%) had LAD obstructive CAD (Table 2).
Full table

Full table
Discussion
The ECG is an important initial diagnostic tool in the evaluation and management of patients presenting with chest pain. ECG findings form the basis on which acute coronary syndromes are classified into STEMI, NSTEMI, or unstable angina, a classification which provides information regarding the extent of myocardium at risk and guides initial therapy.
ST-segment elevation is a sign of transmural ischemia and identifies patients who are likely to benefit from urgent revascularization. The relation between ST-segment elevation on the ECG and the occluded coronary artery has been established in multiple clinical studies in patients with ACS (1). LAD coronary artery obstruction most often results in ST-segment elevation in the precordial leads V1-V4 (anterior, anteroseptal patterns) (1,5). In rare instances, ST-segment elevation in leads V1-V4 signifies RCA occlusion with concomitant right ventricular infarction (1). Isolated ST elevation in leads V4–V6, without ST elevation in leads V1–V3, is usually due to an occlusion of the LCX or distal diagonal branch rather than the main LAD artery (1). ST-segment elevation in leads II, III, and aVF is associated with infarction of the inferior wall (1,5). The culprit vessel in inferior MI may be either the RCA (in the majority of cases) or the LCX artery (5). Consistent with prior studies, we found that all of our patients with anterior STEMI had LAD obstructive CAD, while among patients with inferior STEMI almost 80% had an occluded RCA and all but 1 of the remainder had an occluded LCX artery.
Among patients with NSTEMI, we found that only 53% of patients had definite ECG ischemic abnormalities. This could be due to the fact that we only included patient with single- vessel CAD, while a large percentage of patients with NSTEMI have multivessel CAD. This low percentage could also be explained by the relatively low troponin level we used for inclusion in our study. Other studies have shown higher percentages of ECG changes in NSTEMI, especially if the ECG is obtained during chest pain (6). The ECG in our study was recorded on admission and not necessarily during active chest pain which could explain the lower percentage of ECG changes. However other investigators that included both unstable angina and NSTEMI as in the TIMI registry study reported lower percentages. New ST segment depression >1 mm was present in only 14.3% of 1,416 enrolled patients, isolated T wave inversion in 21.9% of patients. Ischemic ECG changes were present in about 25% patients in a similar study (7,8).
The correlation of ischemic ECG changes with culprit lesion location in NSTEMI has not been extensively reported in the literature (9-11). One study examined ECGs obtained during spontaneous angina or exercise stress testing in patients with angiographically documented 1-vessel CAD. T-wave inversion correctly identified the location of the coronary disease in 84%, and ST depression in 60% of patients (12). In our study, we found that 97% of NSTEMI patients with anterior definite ECG ischemic abnormalities had single vessel LAD disease. This correlation was not as strong for inferior ECG ischemic abnormalities in which 14% of patients did not have RCA or LCX disease but had LAD disease. This discrepancy could be explained to a long wrap-around LAD perfusing part of the inferior wall. An alternative explanation is that inferior wall ECG ischemic abnormalities in NSTEMI are less specific than anterior changes.
The following limitations of this study should be considered. First, we included only patients with 1-vessel CAD, which although it increases accuracy in determining the culprit lesion, makes it less generalizable to the whole NSTEMI population in which multi-vessel CAD is common. Second, we did not analyze ischemic ECG changes by subtype (ST-segment depression vs. T-wave inversion), but rather by the presence or absence of definite ischemic changes overall. Third, the presence or absence of symptoms at the time the ECG was recorded could not be ascertained in this retrospective study. Fourth, the small number of patients with NSTEMI and definite ischemic changes makes for less stable estimates.
Conclusions
In this retrospective study of patients with ischemic symptoms, an elevated troponin I level, and 1-vessel CAD on coronary angiography, only 53% of the NSTEMI patients demonstrated definite ischemic ECG abnormalities. In both STEMI and NSTEMI patients, anterior ischemic changes, when present, were highly predictive of LAD disease. However, inferior ischemic changes were less reliable in predicting the location of the culprit lesion.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was performed with scrupulous attention to ethical principles.
References
- Birnbaum Y, Drew BJ. The electrocardiogram in ST elevation acute myocardial infarction: correlation with coronary anatomy and prognosis. Postgrad Med J 2003;79:490-504. [Crossref] [PubMed]
- Sclarovsky S. editor. Electrocardiography of acute myocardial ischaemic syndromes. London UK: Martin Dunitz Ltd., 1999.
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020-35. [Crossref] [PubMed]
- Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975;51:5-40. [Crossref] [PubMed]
- Zimetbaum PJ, Josephson ME. Use of the electrocardiogram in acute myocardial infarction. N Engl J Med 2003;348:933-40. [Crossref] [PubMed]
- Cohen M, Hawkins L, Greenberg S, et al. Usefulness of ST-segment changes in greater than or equal to 2 leads on the emergency room electrocardiogram in either unstable angina pectoris or non-Q-wave myocardial infarction in predicting outcome. Am J Cardiol 1991;67:1368-73. [Crossref] [PubMed]
- Cannon CP, McCabe CH, Stone PH, et al. The Electrocardiogram Predicts One-Year Outcome of Patients With Unstable Angina and Non–Q Wave Myocardial Infarction: Results of the TIMI III Registry ECG Ancillary Study. J Am Coll Cardiol 1997;30:133-40. [Crossref] [PubMed]
- Nyman I, Areskog M, Areskog NH, et al. Very early risk stratification by electrocardiogram at rest in men with suspected unstable coronary heart disease. The RISC Study Group. J Intern Med 1993;234:293-301. [Crossref] [PubMed]
- Boden WE, O'Rourke RA, Crawford MH, et al. Outcomes in patients with acute non-Q-wave myocardial infarction randomly assigned to an invasive as compared with a conservative management strategy. Veterans Affairs Non-Q-Wave Infarction Strategies in Hospital (VANQWISH) Trial Investigators. N Engl J Med 1998;338:1785-92. [Crossref] [PubMed]
- Kerensky RA, Wade M, Deedwania P, et al. Revisiting the culprit lesion in non-Q-wave myocardial infarction. Results from the VANQWISH trial angiographic core laboratory. J Am Coll Cardiol 2002;39:1456-63. [Crossref] [PubMed]
- Ellestad MH. Stress Testing: Principles and Practice. 4th ed. Philadelphia: Predictive implications, 1996:330-3.
- Fuchs RM, Achuff SC, Grunwald L, et al. Electrocardiographic localization of coronary artery narrowings: studies during myocardial ischemia and infarction in patients with one-vessel disease. Circulation 1982;66:1168-76. [Crossref] [PubMed]


