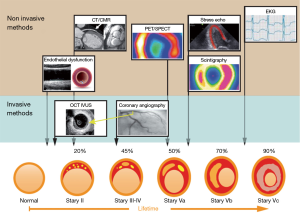
Echocardiographic assessment of myocardial ischemia
Introduction
Since the introduction of “ultrasound cardiography” (echocardiography) by Edler and Hertz (1-3) and the first trial involving the investigation of cardiac wall abnormalities, echocardiography (3) has become the most important and most used tool in cardiology besides electrocardiography (ECG). The first milestones were the detection of abnormal wall motion during angina pectoris (4) and acute myocardial infarction (AMI) (5). The introductions of two-dimensional echocardiography (2DE), pulsed wave (PW)/continuous wave (CW) Doppler techniques, Echo-Contrast (6-8), Trans-esophageal echocardiography (TEE) (9), tissue Doppler imaging (TDI) (10), three-dimensional echocardiography (3DE) (11), and strain echocardiography (strain) (SE) (12) have provided numerous tools with which to manage all conditions related to coronary heart disease (CHD). Additional analyses of diastolic function (13) and myocardial architecture using all possible sonographic technologies (14-16) allow for full functional evaluations of cardiac function.
In the hands of an experienced user, echocardiography can be sufficiently reliable to assess all stages of cardiovascular disease. Further substantiation can be provided by the use of the trans-esophageal approach (17-19). It can be postulated that an experienced cardiologist who is very familiar with echocardiography does not need apply additional techniques, with the exception of supplementation with a Troponin test, in the clinical management of patients with chronic cardiovascular disease (20). Although it sounds quite provocative, this hypothesis is tenable. Cardiovascular magnetic resonance (CMR) (21) and computed tomography (cardio-CT) (22,23) are important tools for ischemic (24) heart disease, but the depth of modern echocardiography (13-15,25-30) and the technical possibilities of advanced echocardiography with the corresponding experience provides many of the answers we need. The use of echocardiography can differ between the USA and Europe. For example, in Germany, cardiologists perform 99% of all examinations themselves. The associated broad clinical expertise provides more options compared with scenarios in which echocardiography is performed by a technician and is based solely on visual expertise. Discussion about the quality improvement process is on-going (31,32).
Echocardiography in the chronic and acute stages of CHD
The physiological response of the myocardial wall to coronary occlusion is the basis for echocardiographic examinations. Tennant and Wiggers were the first to report a reduction in contractility during coronary occlusion (33). This finding was further investigated by Prinzmetal et al. (34). Visualization of a regional decrease in the systolic movement of the endocardium and a decrease in myocardial thickening are the main principles for the diagnosis of myocardial damage (Figure 1). In recent years, an ischemic cascade was suggested (Figure 2) [in the clinical setting, a test for coronary ischemia is not evident in any case what is caused by the different localization of collaterals (35-38)].

2DE provides assessments of the locations and the extent of the acute/chronic stages of CHD (Figure 3) (39) and was introduced as “cross-sectional echocardiography” in 1976 by Feigenbaum et al. (40). The main advantage of this technique is the direct clinical use of this method, for example, as a bedside method in the emergency room and in hospital stations and clinical practice. In patients with thoracic pain, we are able to localize regional wall motion abnormalities using wall motion scores (28) (Figure 1) at rest and diagnose diseases other than CHD (e.g., pericardial effusion, endocarditis, aortic dissection, and valvular disease). In chest pain units, we can evaluate chest pain patients using SE and thus recognize exercise-induced ischemia and evaluate the need for invasive diagnostics. In experienced hands, this technique is a safe and valuable tool (20).
AMI
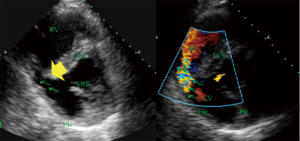
The first description of the use of echocardiography involved the use of M-mode technology during acute infarction (5). Fogelman et al. described wall motion abnormalities during angina pectoris (4). The extent of myocardial ischemia during myocardial infarction should be evaluated by 2DE according to the ASE criteria (28). Additionally, techniques such as PW-Doppler, TDI, strain and 3DE (11) extend the possibilities and supply additional functional or geometrical (11) information, but in the acute phase of infarction, analyses of wall abnormalities or wall structure and valve function via color Doppler provide sufficient clinical information and enables the identification of addition complications of extended infarction, such as inter-ventricular shunts caused by infarction (Figures 4,5), papillary muscle dysfunction and perimyocardial effusion. One of the simplest types of information is that provided by acute measurements of wall motion scores or ejection fractions and analyses of the extent of hypokinesia or akinesia (Figure 1) in terms of the myocardial wall segments. Myocardial contrast echocardiography in AMI (given that the infarction is intracoronary) delineates the area that is at risk for necrosis and the extent of collateral blood flow (41) and aids the evaluation of the microvasculature for “no-reflow” areas. Serial intravenous myocardial contrast echocardiography has the potential to identify patients who are likely to exhibit improved left ventricular function after AMI and can be used to define perfusion defects (42).
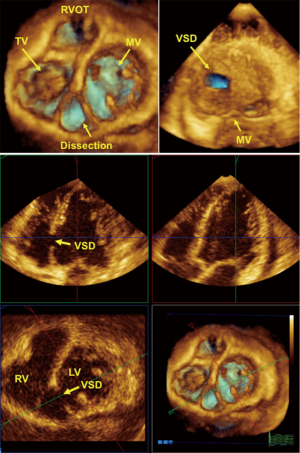
Chronic stages of ischemic coronary disease
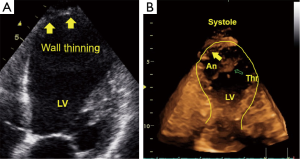
Ventricular remodeling after myocardial infarction is a primary issue in echocardiography (43). The management of patients in the chronic stages of ischemic heart disease requires the following important information: (I) global and regional contractile function of the heart at rest; (II) valvular function; and (III) local complications, such as mural thrombi, myocardial scars and ventricular septal defects. Information concerning these three points can be provided by basic 2DE. Using additional techniques, such as PW Doppler, color Doppler or TDI/strain or 3DE, we are able to perform full conservative assessments of cardiac function, including right ventricle (RV) and left ventricle (LV) function, to manage the patient without the complications of an infarction and manage patients with heart failure in all stages of the disease. After an acute infarction, it may be important to know whether the patient develops an apical LV aneurysm (Figure 3, which usually occurs after anterior myocardial infarction). This type of aneurysm exhibits greater hemodynamically effects than inferior/posterior aneurysms. Early reperfusion strategies in many countries with 24-h PCI-attendance reduce the development of LV aneurysms. Using all views (including the subcostal echo window) and all lying positions of the patient, it is possible to obtain echocardiograms that enable sufficient visual evaluation and clinical management that reaches 100%. Under these conditions and managements, obese patients can also be examined echocardiographically. Indeed, we have experienced greater difficulties with patients with severe chronic pulmonary disease.
SE
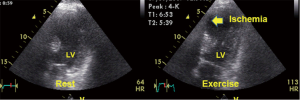
In the last 35 years, since one of the first descriptions of two-dimensional stress echocardiography (2D-SE) by Wann et al. (44), 2D-SE has considerably developed. This method is cost effective, safe and free of non-ionizing radiation (32). SE is one of the most important tools for assessing myocardial ischemia (20,29,45) and viable myocardia (46,47), in addition being easy to use at the bedside (45,48). Developments in digital hardware and software and improvements in ultrasound techniques have yielded excellent results from SE regarding scanning for ischemia (Figure 6) and risk assessment (45,49-52), screening after PTCA (53) and testing for anti-ischemic drug effects (54). Recent studies have demonstrated the advantages of exercise echocardiography from the prognostic perspective in patients with known and unknown CHD (20) and left bundle brunch block (55). In patients who have undergone non-surgical revascularization (56), studies have demonstrated that analyses of quantitative parameters determined by exercise echocardiography increase the sensitivity of the diagnosis of restenosis (57).
SE can be performed using physical exercise (e.g., bicycles and treadmills), atrial pacing, and various drugs, such as dobutamine, dipyridamole and adenosine. The sensitivity of catecholamine SE is lower than that of peak exercise echocardiography, but dobutamine SE is technically simpler and can be performed in patients who are unable to exercise. The limitations of pharmacological stress are adverse side effects and the more time-consuming stress procedure. In assessments of patients with CHD, SE has been demonstrated to be valuable for diagnosis and prognosis (20). Furthermore, there are different stress modalities (in particular, low-dose dobutamine stress) for the detection of viable myocardium (46). Despite the high reported accuracy of exercise echocardiography in the diagnosis of CHD, the sensitivity and specificity can be affected by multiple factors (58,59). In most cases, decreased accuracy is caused by suboptimal image quality and decreased detection of the endocardial border. The diagnostic accuracy of SE depends on the type of stress procedure utilized, the digital processing technique and the experience of the investigator (59). The inter-institutional observer variance in the interpretation of SE varies from agreements of 100% to 43% in the lowest image quality stress echocardiograms (58). Presently, we have to decide which technique we should select as the gatekeeper to cardiac catheterization (60). It can be suggested that in each clinical situation, we need to select the technique and stress method for which we have the optimal personal/individual expertise (59), and we have to consider the economic and biological costs of all cardiac imaging techniques (61).
Novel techniques and screening for ischemia
In recent years, ultrasound technology has developed rapidly with continuous improvements in image quality and the use of new approaches (e.g., TDI, strain, contrast echocardiography, and 3DE). Thus, new ischemic indicators need to be identified (12,29,62-65). TDI has provided new insight into the analysis of myocardial mechanics in patients with coronary disease (10,62,66). The clinical utility of the TDI technology has offered new insight into the analysis of LV dyssynchrony (67,68), and among the most frequently used applications are the evaluation of diastolic function (13,69,70) and the appraisal of LV end-diastolic pressure (71) (E/e’ ratio) (72). The last issue remains controversial (73).
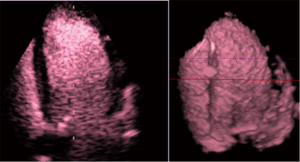
Contrast echocardiography (Figure 7) has been well introduced to the clinical and scientific settings (6,74,75) and has a safe place regarding volume diagnostics and improvements in endocardial border visualization (76-78). The technique can be recommended for patients with poor acoustic windows and is applicable when CMR cannot be performed (79).
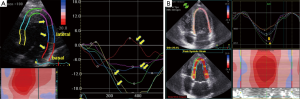
Strain-technology (speckle tracking imaging) was introduced in 1998 by Heimdal et al. (12) to determine the deformation or strain of the myocardial tissue. The rate of this deformation, i.e., the strain rate, is equivalent to the myocardial velocity gradient. The strain rate has been described as equivalent to the shortening velocity per fiber length (12). It’s postulated that it can also be used to evaluate the function of the ventricular fiber architecture (80,81) and to measure dynamic changes in the geometry and fibrous structure of the heart (82). Acute systolic and diastolic changes during angioplasty could potentially be evaluated using strain and strain rate measurements (83) as well as early changes in myocardial function in hypertensive patients could be detected (30). The first announcements about the use of strain-technology in CHD were published by Voigt et al. (84,85). The first clinical use was suggested as early as 2003 (86), and today, further standards for the analyses of left myocardial function are being proposed (65). The use of apical views for the evaluation of cardiac deformation seems to be clinically useful (87), and apical views result in the best reproducibility (88). Analyses of systolic regional deformations (Figure 8), the diastolic properties in CHD, estimations of LV filling and left atrial function (73) are further advantages of this technology. The value of myocardial deformation imaging using speckle tracking echocardiography (strain) has been suggested to be comparable with that of CMR (89). We are looking forward to new comprehensive research with new technologies and equipment with better resolution to achieve reliable and safe diagnoses in myocardial ischemia using echocardiography.
Pitfalls and limitations of diagnosing ischemia via echocardiography
Basically, the diagnosis of ischemia using echocardiography is safe and has a secure place in the management of stable angina pectoris (90). However, one constant problem is reproducibility (58,77,88). Additional second problem is the “low volume” of SE examinations by a single operator in clinical practice (32). In the UK, only 28.2% of operators performed >500 procedures/year. At the frame rates of 3DE and SE, spatial and temporal resolution are problems. In general, when 3DE is used for stress examination, if the heart rate is >110 beats/min [we need >130 beats/min from the prognostic perspective (20)], the acquisition of a sufficient number of volumes per heart cycle becomes a problem. An additional problem can be the acoustic window for echocardiography when a safe answer for decision making regarding interventional procedures is needed (91,92). In these cases, an additional imaging technology, such as CMR or single-photon emission computed tomography (SPECT), is needed. It seems that echo contrast improves endocardial border detection and can improve the reproducibility of LV volume estimation (77). The intravenous injection of contrast agent improves the opacification of the myocardial border, but the analysis of myocardial perfusion remains problematic (78). Even after 20 years of research, echocardiographic perfusion analysis is not sufficiently reliable (and perhaps too expensive) and has failed to be implemented in daily routines despite some reports have demonstrated opposite results (6,74,91,93). Myocardial perfusion analysis using CMR and SPECT seems to be more reliable and safer (24,90,91).
Conclusions and insights for the future
When using echocardiography to assess ischemia, the results obtained at rest vs. those obtained during exercise need to be differentiated. We suggest that, regarding resting assessments, echocardiography combined with all of the new developments (e.g., 3DE, harmonic imaging, TDI, strain, and contrast echocardiography) offers at least as many advantages (and can be nearly as reliable) as CMR when conducted by experienced operators, given that it is possible to obtain individual acoustic windows and a regional evaluation of the myocardium. For exercise assessments, there is a resolution limitation that is caused by non-sufficient scanning conditions in nearly 5% of patients, and for these patients, additional imaging techniques should be recommended. When using echocardiography to evaluate cardiovascular disease, we need to consider the following issues:
- Daily clinical use;
- Institutional/personal availability (in terms of costs, education, and equipment);
- Principal technical opportunities;
- Research situations and the “pressure to publish”.
Referring to (I): for daily use, we need and have a simple, reliable, safe situation for the use of 2DE (a high-end machine) in combination with Doppler/TDI/strain/3DE and contrast echocardiography for use in clinical decision-making.
Referring to (II): it is necessary to become an expert and to strive for improvement even after 30 years of experience. Additionally, there is a need for high-quality equipment and intensive training of the operator with continuous updates regarding new developments in the field.
Referring to (III): the technical opportunities for high-end sonographic devices are great, and these opportunities combined with workstations are rarely ever fully exploited in daily routines or even in research. This issue must be considered by cardiologists in their daily work. Otherwise, there is a risk of becoming frustrated due to failure in reaching the goals presented in presentations and publications.
Referring to (IV): work on new developments and with new equipment is always exciting and stimulating. Only due to this enthusiasm were all of the following new technologies introduced into daily practice: 2DE, color Doppler, SE, TDI, 3DE, and Strain. This enthusiasm persisted despite all of the initial attempts to use the new technologies being called “crazy”. Echocardiography is an excellent tool for the diagnosis of acute, chronic and exercise-induced ischemia. The critical use of echocardiography is recommended (94). In brief, it is necessary to use echocardiography in the assessment of CHD, and it is important to gain solid expertise with the related techniques (59).
Acknowledgements
Thanks to Frank Blumberg (Multimedias) for graphical design of Figure 2.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Feigenbaum H. Evolution of echocardiography. Circulation 1996;93:1321-7. [Crossref] [PubMed]
- Acierno LJ, Worrell LT. Inge Edler: father of echocardiography. Clin Cardiol 2002;25:197-9. [Crossref] [PubMed]
- Edler I, Hertz CH. The use of ultrasonic reflectoscope for the continuous recording of the movements of heart walls. Kungl. Fysiografiska sällskapets i Lund förhandlingar 1954;24:1-19.
- Fogelman AM, Abbasi AS, Pearce ML, et al. Echocardiographic study of the abnormal motion of the posterior left ventricular wall during angina pectoris. Circulation 1972;46:905-13. [Crossref] [PubMed]
- Inoue K, Smulyan H, Mookherjee S, et al. Ultrasonic measurement of left ventricular wall motion in acute myocardial infarction. Circulation 1971;43:778-85. [Crossref] [PubMed]
- Kaufmann BA, Wei K, Lindner JR. Contrast echocardiography. Curr Probl Cardiol 2007;32:51-96. [Crossref] [PubMed]
- Leischik R, Beller KD, Erbel R. Comparison of a new intravenous echo contrast agent (BY 963) with Albunex for opacification of left ventricular cavity. Basic Res Cardiol 1996;91:101-9. [PubMed]
- Dijkmans PA, Senior R, Becher H, et al. Myocardial contrast echocardiography evolving as a clinically feasible technique for accurate, rapid, and safe assessment of myocardial perfusion: the evidence so far. J Am Coll Cardiol 2006;48:2168-77. [Crossref] [PubMed]
- Erbel R. Transesophageal echocardiography. New window to coronary arteries and coronary blood flow. Circulation 1991;83:339-41. [Crossref] [PubMed]
- Erbel R, Wallbridge DR, Zamorano J, et al. Tissue Doppler echocardiography. Heart 1996;76:193-6. [Crossref] [PubMed]
- Buck T, Schön F, Baumgart D, et al. Tomographic left ventricular volume determination in the presence of aneurysm by three-dimensional echocardiographic imaging. I: Asymmetric model hearts. J Am Soc Echocardiogr 1996;9:488-500. [Crossref] [PubMed]
- Heimdal A, Støylen A, Torp H, et al. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr 1998;11:1013-9. [Crossref] [PubMed]
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277-314. [Crossref] [PubMed]
- Zhou X, Thavendiranathan P, Chen Y, et al. Feasibility of Automated Three-Dimensional Rotational Mechanics by Real-Time Volume Transthoracic Echocardiography: Preliminary Accuracy and Reproducibility Data Compared with Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2016;29:62-73. [Crossref] [PubMed]
- Barros MV, Leren IS, Edvardsen T, et al. Mechanical Dispersion Assessed by Strain Echocardiography Is Associated with Malignant Arrhythmias in Chagas Cardiomyopathy. J Am Soc Echocardiogr 2016;29:368-74. [Crossref] [PubMed]
- Papadacci C, Tanter M, Pernot M, et al. Ultrasound backscatter tensor imaging (BTI): analysis of the spatial coherence of ultrasonic speckle in anisotropic soft tissues. IEEE Trans Ultrason Ferroelectr Freq Control 2014;61:986-96. [Crossref] [PubMed]
- Deutsch HJ, Curtius JM, Leischik R, et al. Diagnostic value of transesophageal echocardiography in cardiac surgery. Thorac Cardiovasc Surg 1991;39:199-204. [Crossref] [PubMed]
- Leischik R, Curtius JM, Deutsch HJ, et al. Advantages of biplane transesophageal echocardiography. Z Kardiol 1990;79:850-7. [PubMed]
- Deutsch HJ, Curtius JM, Leischik R, et al. Reproducibility of assessment of left-ventricular function using intraoperative transesophageal echocardiography. Thorac Cardiovasc Surg 1993;41:54-8. [Crossref] [PubMed]
- Leischik R, Dworrak B, Littwitz H, et al. Prognostic significance of exercise stress echocardiography in 3329 outpatients (5-year longitudinal study). Int J Cardiol 2007;119:297-305. [Crossref] [PubMed]
- Henn MC, Cupps BP, Kar J, et al. Quantifying "normalized" regional left ventricular contractile function in ischemic coronary artery disease. J Thorac Cardiovasc Surg 2015;150:240-6. [Crossref] [PubMed]
- Doh JH, Koo BK, Nam CW, et al. Diagnostic value of coronary CT angiography in comparison with invasive coronary angiography and intravascular ultrasound in patients with intermediate coronary artery stenosis: results from the prospective multicentre FIGURE-OUT (Functional Imaging criteria for GUiding REview of invasive coronary angiOgraphy, intravascular Ultrasound, and coronary computed Tomographic angiography) study. Eur Heart J Cardiovasc Imaging 2014;15:870-7. [Crossref] [PubMed]
- McClelland RL, Jorgensen NW, Budoff M, et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol 2015;66:1643-53. [Crossref] [PubMed]
- Sechtem U, Tanner FC, Gaemperli O. The year in cardiology 2013: imaging in ischaemic heart disease. Eur Heart J 2014;35:344-8. [Crossref] [PubMed]
- Yang LT, Nagata Y, Otani K, et al. Feasibility of One-Beat Real-Time Full-Volume Three-Dimensional Echocardiography for Assessing Left Ventricular Volumes and Deformation Parameters. J Am Soc Echocardiogr 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Uusitalo V, Luotolahti M, Pietilä M, et al. Two-Dimensional Speckle-Tracking during Dobutamine Stress Echocardiography in the Detection of Myocardial Ischemia in Patients with Suspected Coronary Artery Disease. J Am Soc Echocardiogr 2016;29:470-479.e3. [Crossref] [PubMed]
- Kutty S, Xiao Y, Olson J, et al. Safety and Efficacy of Cardiac Ultrasound Contrast in Children and Adolescents for Resting and Stress Echocardiography. J Am Soc Echocardiogr 2016;29:655-62. [Crossref] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1-39.e14. [Crossref] [PubMed]
- Leischik R, Bartel T, Möhlenkamp S, et al. Stress echocardiography: new techniques. Eur Heart J 1997;18 Suppl D:D49-56.
- Hensel KO, Jenke A, Leischik R. Speckle-tracking and tissue-Doppler stress echocardiography in arterial hypertension: a sensitive tool for detection of subclinical LV impairment. Biomed Res Int 2014;2014:472562.
- Gilliland YE, Lavie CJ, Ahmad H, et al. Development and Implementation of a Quality Improvement Process for Echocardiographic Laboratory Accreditation. Echocardiography 2016;33:459-71. [Crossref] [PubMed]
- Bhattacharyya S, Chehab O, Khattar R, et al. Stress echocardiography in clinical practice: a United Kingdom National Health Service Survey on behalf of the British Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2014;15:158-63. [Crossref] [PubMed]
- Tennant R, Wiggers CJ. The effect of coronary occlusion on myocardial contraction. American Journal of Physiology--Legacy Content 1935;112:351-61.
- Prinzmetal M, Schwartz LL, Corday E, et al. Studies on the coronary circulation; loss of myocardial contractility after coronary artery occlusion. Ann Intern Med 1949;31:429-49. [Crossref] [PubMed]
- Erbel R, Budoff M. Improvement of cardiovascular risk prediction using coronary imaging: subclinical atherosclerosis: the memory of lifetime risk factor exposure. Eur Heart J 2012;33:1201-13. [Crossref] [PubMed]
- Erbel R. The dawn of a new era--non-invasive coronary imaging. Herz 1996;21:75-7. [PubMed]
- Sigwart U, Grbic M, Payot M, et al. Ischemic events during coronary artery balloon obstruction. In: W Rutishauser, H Roskamm, editors. Silent Myocardial Ischemia. New York: Springer Berlin Heidelberg, 1984:29-36.
- Nesto RW, Kowalchuk GJ. The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia. Am J Cardiol 1987;59:23C-30C. [Crossref] [PubMed]
- Heger JJ, Weyman AE, Wann LS, et al. Cross-sectional echocardiography in acute myocardial infarction: detection and localization of regional left ventricular asynergy. Circulation 1979;60:531-8. [Crossref] [PubMed]
- Feigenbaum H, Corya BC, Dillon JC, et al. Role of echocardiography in patients with coronary artery disease. Am J Cardiol 1976;37:775-86. [Crossref] [PubMed]
- Villanueva FS. Myocardial contrast echocardiography in acute myocardial infarction. Am J Cardiol 2002;90:38J-47J. [Crossref] [PubMed]
- Lepper W, Hoffmann R, Kamp O, et al. Assessment of myocardial reperfusion by intravenous myocardial contrast echocardiography and coronary flow reserve after primary percutaneous transluminal coronary angioplasty correction of angiography in patients with acute myocardial infarction. Circulation 2000;101:2368-74. [Crossref] [PubMed]
- Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990;81:1161-72. [Crossref] [PubMed]
- Wann LS, Faris JV, Childress RH, et al. Exercise cross-sectional echocardiography in ischemic heart disease. Circulation 1979;60:1300-8. [Crossref] [PubMed]
- Picano E. Stress echocardiography. From pathophysiological toy to diagnostic tool. Circulation 1992;85:1604-12. [Crossref] [PubMed]
- Barilla F, Gheorghiade M, Alam M, et al. Low-dose dobutamine in patients with acute myocardial infarction identifies viable but not contractile myocardium and predicts the magnitude of improvement in wall motion abnormalities in response to coronary revascularization. Am Heart J 1991;122:1522-31. [Crossref] [PubMed]
- Hoffer EP, Dewé W, Celentano C, et al. Low-level exercise echocardiography detects contractile reserve and predicts reversible dysfunction after acute myocardial infarction: comparison with low-dose dobutamine echocardiography. J Am Coll Cardiol 1999;34:989-97. [Crossref] [PubMed]
- Senior R, Kenny A, Nihoyannopoulos P. Stress echocardiography for assessing myocardial ischaemia and viable myocardium. Heart 1997;78 Suppl 1:12-8. [Crossref] [PubMed]
- Salustri A, Pozzoli MM, Reijs AE, et al. Comparison of exercise echocardiography with myocardial perfusion scintigraphy for the diagnosis of coronary artery disease. Herz 1991;16:388-94. [PubMed]
- Ryan T, Vasey CG, Presti CF, et al. Exercise echocardiography: detection of coronary artery disease in patients with normal left ventricular wall motion at rest. J Am Coll Cardiol 1988;11:993-9. [Crossref] [PubMed]
- Nerlekar N, Mulley W, Rehmani H, et al. Feasibility Of Exercise Stress Echocardiography For Cardiac Risk Assessment In Chronic Kidney Disease Patients Prior To Renal Transplantation. Clin Transplant 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Leischik R, Erbel R. Prognostic value of stress echocardiography. Herz 2005;30:743-53. [Crossref] [PubMed]
- Fioretti PM, Pozzoli MM, Ilmer B, et al. Exercise echocardiography versus thallium-201 SPECT for assessing patients before and after PTCA. Eur Heart J 1992;13:213-9. [PubMed]
- Leischik R, Adamczewski O, Pötter S, et al. Stress echocardiography--a new test for evaluating the anti-ischemic effect of medication. Z Kardiol 1995;84:621-32. [PubMed]
- Bouzas-Mosquera A, Peteiro J, Alvarez-García N, et al. Prognostic value of exercise echocardiography in patients with left bundle branch block. JACC Cardiovasc Imaging 2009;2:251-9. [Crossref] [PubMed]
- Mertes H, Erbel R, Nixdorff U, et al. Exercise echocardiography for the evaluation of patients after nonsurgical coronary artery revascularization. J Am Coll Cardiol 1993;21:1087-93. [Crossref] [PubMed]
- Dori G, Denekamp Y, Fishman S, et al. Exercise stress testing, myocardial perfusion imaging and stress echocardiography for detecting restenosis after successful percutaneous transluminal coronary angioplasty: a review of performance. J Intern Med 2003;253:253-62. [Crossref] [PubMed]
- Hoffmann R, Lethen H, Marwick T, et al. Analysis of interinstitutional observer agreement in interpretation of dobutamine stress echocardiograms. J Am Coll Cardiol 1996;27:330-6. [Crossref] [PubMed]
- Picano E, Lattanzi F, Orlandini A, et al. Stress echocardiography and the human factor: the importance of being expert. J Am Coll Cardiol 1991;17:666-9. [Crossref] [PubMed]
- Marwick TH, Cho I. Finding the Gatekeeper to the Cardiac Catheterization Laboratory: Coronary CT Angiography or Stress Testing? J Am Coll Cardiol 2015;65:2747-56. [Crossref] [PubMed]
- Picano E. Economic and biological costs of cardiac imaging. Cardiovasc Ultrasound 2005;3:13. [Crossref] [PubMed]
- Sutherland GR, Stewart MJ, Groundstroem KW, et al. Color Doppler myocardial imaging: a new technique for the assessment of myocardial function. J Am Soc Echocardiogr 1994;7:441-58. [Crossref] [PubMed]
- D'hooge J, Heimdal A, Jamal F, et al. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr 2000;1:154-70. [Crossref] [PubMed]
- Mada RO, Duchenne J, Voigt JU. Tissue Doppler, strain and strain rate in ischemic heart disease "how I do it". Cardiovasc Ultrasound 2014;12:38. [Crossref] [PubMed]
- Voigt JU, Pedrizzetti G, Lysyansky P, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 2015;16:1-11. [Crossref] [PubMed]
- Drozdz J, Erbel R. Ischemic Heart Disease. In: Erbel R, Nesser HJ, Drozdz J, editors. Atlas of Tissue Doppler Echocardiography (TDE). Darmstadt: Steinkopff-Verlag Heidelberg, 1995:69-90.
- Bruch C, Schmermund A, Leischik R, et al. Detection of ventricular asynchrony using tissue Doppler echocardiography--a new method for assessing left ventricular wall motion. Z Kardiol 1997;86:827-38. [Crossref] [PubMed]
- Risum N, Sogaard P, Hansen TF, et al. Comparison of dyssynchrony parameters for VV-optimization in CRT patients. Pacing Clin Electrophysiol 2013;36:1382-90. [Crossref] [PubMed]
- Kim HL, Zo JH, Seo JB, et al. Additional value of lateral tissue Doppler imaging in the assessment of diastolic dysfunction among subjects with pseudonormal pattern of mitral inflow. Cardiovasc Ultrasound 2013;11:31. [Crossref] [PubMed]
- Hayashi SY, Lind BI, Seeberger A, et al. Analysis of mitral annulus motion measurements derived from M-mode, anatomic M-mode, tissue Doppler displacement, and 2-dimensional strain imaging. J Am Soc Echocardiogr 2006;19:1092-101. [Crossref] [PubMed]
- Sugimoto T, Dohi K, Tanabe M, et al. Echocardiographic estimation of pulmonary capillary wedge pressure using the combination of diastolic annular and mitral inflow velocities. J Echocardiogr 2013;11:1-8. [Crossref] [PubMed]
- Galderisi M, Rapacciuolo A, Esposito R, et al. Site-dependency of the E/e' ratio in predicting invasive left ventricular filling pressure in patients with suspected or ascertained coronary artery disease. Eur Heart J Cardiovasc Imaging 2013;14:555-61. [Crossref] [PubMed]
- Leischik R, Littwitz H1, Dworrak B, et al. Echocardiographic Evaluation of Left Atrial Mechanics: Function, History, Novel Techniques, Advantages, and Pitfalls. Biomed Res Int 2015;2015:765921.
- Senior R, Dwivedi G, Hayat S, et al. Clinical benefits of contrast-enhanced echocardiography during rest and stress examinations. Eur J Echocardiogr 2005;6 Suppl 2:S6-13. [Crossref] [PubMed]
- Gibson PH, Becher H, Choy JB. The current state of myocardial contrast echocardiography: what can we read between the lines? Eur Heart J Cardiovasc Imaging 2014;15:351. [Crossref] [PubMed]
- Nemes A, Geleijnse ML, Krenning BJ, et al. Usefulness of ultrasound contrast agent to improve image quality during real-time three-dimensional stress echocardiography. Am J Cardiol 2007;99:275-8. [Crossref] [PubMed]
- Leischik R, Kuhlmann C, Bruch C, et al. Reproducibility of stress echocardiography using intravenous injection of ultrasound contrast agent (BY 963). Int J Card Imaging 1997;13:387-94. [Crossref] [PubMed]
- Leischik R, Rose J, Caspari G, et al. Contrast echocardiography for assessment of myocardial perfusion. Herz 1997;22:40-50. [Crossref] [PubMed]
- Saloux E, Labombarda F, Pellissier A, et al. Diagnostic value of three-dimensional contrast-enhanced echocardiography for left ventricular volume and ejection fraction measurement in patients with poor acoustic windows: a comparison of echocardiography and magnetic resonance imaging. J Am Soc Echocardiogr 2014;27:1029-40. [Crossref] [PubMed]
- Greenbaum RA, Ho SY, Gibson DG, et al. Left ventricular fibre architecture in man. Br Heart J 1981;45:248-63. [Crossref] [PubMed]
- Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr 2011;12:167-205. [Crossref] [PubMed]
- Nielsen PM, Le Grice IJ, Smaill BH, et al. Mathematical model of geometry and fibrous structure of the heart. Am J Physiol 1991;260:H1365-78. [PubMed]
- Kukulski T, Jamal F, D'Hooge J, et al. Acute changes in systolic and diastolic events during clinical coronary angioplasty: a comparison of regional velocity, strain rate, and strain measurement. J Am Soc Echocardiogr 2002;15:1-12. [Crossref] [PubMed]
- Voigt JU, Lindenmeier G, Exner B, et al. Incidence and characteristics of segmental postsystolic longitudinal shortening in normal, acutely ischemic, and scarred myocardium. J Am Soc Echocardiogr 2003;16:415-23. [Crossref] [PubMed]
- Voigt JU, Exner B, Schmiedehausen K, et al. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation 2003;107:2120-6. [Crossref] [PubMed]
- Yip G, Abraham T, Belohlavek M, et al. Clinical applications of strain rate imaging. J Am Soc Echocardiogr 2003;16:1334-42. [Crossref] [PubMed]
- Feigenbaum H, Mastouri R, Sawada S. A practical approach to using strain echocardiography to evaluate the left ventricle. Circ J 2012;76:1550-5. [Crossref] [PubMed]
- Leischik R, Dworrak B, Hensel K. Intraobserver and interobserver reproducibility for radial, circumferential and longitudinal strain echocardiography. Open Cardiovasc Med J 2014;8:102-9. [Crossref] [PubMed]
- Altiok E, Tiemann S, Becker M, et al. Myocardial deformation imaging by two-dimensional speckle-tracking echocardiography for prediction of global and segmental functional changes after acute myocardial infarction: a comparison with late gadolinium enhancement cardiac magnetic resonance. J Am Soc Echocardiogr 2014;27:249-57. [Crossref] [PubMed]
- Task Force Members, Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949-3003. [Crossref] [PubMed]
- Senior R, Moreo A, Gaibazzi N, et al. Comparison of sulfur hexafluoride microbubble (SonoVue)-enhanced myocardial contrast echocardiography with gated single-photon emission computed tomography for detection of significant coronary artery disease: a large European multicenter study. J Am Coll Cardiol 2013;62:1353-61. [Crossref] [PubMed]
- Shah BN, Senior R. Stress echocardiography in patients with morbid obesity. Echo Res Pract 2016;3:R13-8. [Crossref] [PubMed]
- Bhatia VK, Senior R. Contrast echocardiography: evidence for clinical use. J Am Soc Echocardiogr 2008;21:409-16. [Crossref] [PubMed]
- Byrd BF 3rd, Abraham TP, Buxton DB, et al. A Summary of the American Society of Echocardiography Foundation Value-Based Healthcare: Summit 2014: The Role of Cardiovascular Ultrasound in the New Paradigm. J Am Soc Echocardiogr 2015;28:755-69. [Crossref] [PubMed]