Resistant mechanisms to BRAF inhibitors in melanoma
Introduction
Melanoma is the most aggressive form of skin cancer, representing over 10% of all skin cancers, but responsible for more than 80% of skin cancer-related deaths (1).
The mitogen-activated protein kinases (MAPK) pathway is a key oncogenic signaling system of a relay of kinases that culminate in cell proliferation, differentiation and survival. Genomic classification of cutaneous melanoma proposed four subtypes: BRAF mutations, NRAS mutation, loss of NF-1 and triple wild-type.
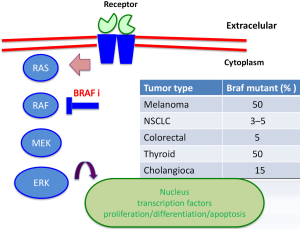
The discovery of hotspot mutations in BRAF V600E, a key serine-threonine kinase in the RAS-RAF-MEK-ERK (MAPK pathway) signaling pathway, led to development of molecular targeted therapies for melanoma (2). Activating BRAF mutations harbor 50% of cutaneous melanoma with non-chronic sun damage (involves another tumor as colorectal cancer, ovarian, thyroid) (Figure 1). In other clinical subtypes of melanoma, BRAF mutations are present in 10–20% of mucosal or acral melanoma, but absent in uveal melanoma (3,4). The most common mutation is a substitution of valine to glutamic acid (V600E) or lysine (V600K) at codon 600 in 20% of BRAF-mutants patients (5). The BRAF V600R occurs in 7% of patients, presents a substitution of valine to arginine. Mutations in NRAS are the second most common genetic alteration, being present in 20% of melanomas, and always exclusive to BRAF mutations (6). Twenty to thirty percent of mucosal melanomas harbor mutations or genomic amplification of cKIT (7). These are infrequently altered in cutaneous melanoma, while more than 85% of uveal melanomas contains mutations in GNAQ and GNA11; these mutations are also rarely present in cutaneous melanoma (8,9). BRAF inhibitors (vemurafenib, dabrafenib) and MEK inhibitors (trametinib) have been approved for treatment of unresectable or metastatic BRAF mutated melanoma, since they have shown improved progression-free survival (PFS) and overall survival (OS) compared to chemotherapy (10). Although responses and tumor control with BRAF inhibitors are impressive, durability of response is limited due to resistance, and evidence of disease progression can be seen within 6 to 8 months of starting therapy due to development of resistance mechanisms (11,12). Combined therapy with BRAF and MEK inhibitors has shown benefit in PFS and response rate, compared with monotherapy, delaying the appearance of alterations involved in resistance (13).
Mechanisms of resistance to MAPK pathway inhibition can be subdivided in two groups: MAPK–dependent and MAPK-independent. Into BRAF/MEK/ERK dependent reactivation, mechanisms of resistance including: amplification of BRAF, splicing BRAF, NRAS mutation, MEK mutation, loss of NF1. MAPK-independent includes: up-regulated receptor tyrosine kinases (RTKS), overexpression COT.
Primary and acquired resistance, tumoral heterogeneity
Numerous mechanisms of resistance have been detected using in vitro and in vivo models, and many have been observed in pre- and post-treatment tumor samples. It is very difficult to explain the behavior of neoplastic cells, but melanoma cells are highly heterogeneous, regardless of their mutational and epigenomic profile. Resistant melanoma cells may become so under selective pressure from therapies from preexisting resistant clones, or secondary as an evolving process during treatment. Melanoma cells do not show arrangement, but express great plasticity, with several tumor subclones sustained by the microenvironment. This microenvironment supports tumor growth and the maintenance of two populations, slow-cycling tumor cells, and cells with epithelial to mesenchymal transition (EMT). Plasticity supports organization within the tumor, and survival during treatment with BRAF inhibitors in vitro and in vivo (3–6% complete response) (14).
These mechanisms are known as primary or intrinsic resistance when no clinical benefit is achieved, and as secondary or acquired when progressive disease is seen after clinical benefit. Mechanisms of primary resistance include mutations in RAC1, loss of PTEN, amplification of cyclin D; secondary resistance mechanisms include alternative splicing of BRAF, BRAF copy number amplification and alterations in PI3K (Table 1).

Full table
Mechanisms of primary resistance
Loss of PTEN
Loss of PTEN occurs in 10–35% of melanomas, is mutually exclusive to NRAS mutations and coexists with BRAF mutations. PTEN is lost in the most of melanomas by loss of heterozygosity, mutations, and methylation. PTEN serves a tumor suppressor and major regulator of PI3K (15,16). Deletions or mutations in PTEN are associated with shorter PFS in patients treated with BRAF inhibitors. It is known, that PTEN loss alone is not sufficient to confer resistance to BRAF inhibitors other concurrent alterations, such as activation of AKT, are necessary. Cell lines with inactivation of PTEN are less sensitive to BRAF inhibitors than wild-type PTEN melanoma cells (17). In clinical practice, patients with wild-type PTEN treated with BRAF inhibitors had longer PFS than patients with mutated PTEN (18) (32.1 vs. 18 weeks; P=0.066), and a weak association was seen between low expression of PTEN and lower response rates in patients treated with BRAF inhibitors (19). Dual inhibition BRAF and PI3K has been studied as a means of overcoming this resistance and restoring apoptosis in deleted PTEN cells (20).
Dysregulation of cyclin-dependent kinase 4 (CDK4)
In the cell cycle, cyclin D1 regulates proliferation through binding to CDK4 and CDK6, which activate retinoblastoma protein and lead to cell cycle progression. CDK4 mutations and cyclin D1 amplification confer strong resistance to therapy with BRAF inhibitors (21). Cyclin D1 amplifications are found in about 20% of BRAF mutated melanomas. CDK4-6 inhibitors (key regulators of G1-S transition of the cell cycle) alone failed to decrease tumor size, but when BRAF and MEK inhibitors were combined, complete responses were achieved in 30% of mouse models (22).
Hepatocyte grow factor (HGF) and microenvironment
Stromal cells secrete several factors such as HGF receptor c-MET, able to activate tumor cell growth in a paracrine form upregulating PI3K, thus conferring resistance to BRAF inhibitors or combinations of BRAF and MEK inhibitors (23). It was reported in cell lines that the combination of BRAF and AKT inhibitors or anti-MET therapies can lead to overcoming resistance of this pathway (24).
Loss of NF1
NF1 is a tumor suppressor of RAS; mutations of NF-1 are present in 14% of melanomas. Inactivation of NF1 leads to activation of RAS, PI3K-AKT-mTOR and MAPK pathways. NF1 mutations prevents under BRAF inhibition senescence of melanoma cells and too, NF1 mutations and NRAS mutations coexist in the inactivating BRAF, were been required RAS isoforms for the pro-tumorigenic activity of these cells. In this scenario, one means of overcoming resistance to BRAF inhibition is the combination of MEK and mTOR inhibitors (25).
RAC1 mutations
RAC1 is a key regulator of motility and proliferation cells and a GTPase effector of RAS. RAC mutations are present in 9% of melanomas, coexisting with BRAF and NRAS mutations. In clinical practice, from a cohort of 45 patients treated with BRAF inhibitors, 14 showed primary resistance, three had RAC1 mutations, any of the which no patients with reached response to therapy (26,27).
Mechanisms of secondary resistance
Acquired resistance mechanisms are associated mainly with the reactivation of the MAP kinase pathway (>70%), sometimes it coexists with the reactivation of the PI3K-AKT pathway, in a less percentage of patients it depends in exclusive on the AKT reactivation in parallel (PI3K-PTEN-mTOR).
BRAF variants
BRAF inhibitors drive suppression MEK/ERK signaling, although they activate MEK/ERK signaling in RAS mutant cells. In the presence of oncogene RAS, BRAF inhibitors lead to the formation of CRAF-BRAF heterodimers or homodimers. One part of BRAF inhibitors bound to hetero/homodimer, and another part that is drug-free. The BRAF inhibitor bound leads to activation of the drug-free, and through conformational changes, activating CRAF, and finally MEK-ERK activation. To overcome this resistance have been tested the combination targeting of BRAF and MEK inhibitors.
To date, no secondary gatekeeper BRAF mutations have been found. Two aberrations have been described affecting BRAF gene: gene copy number gain or amplification of BRAF, and alternative splicing of BRAF. Amplification of BRAF is a copy gain of the mutant allele of BRAF, resulting in overexpression and leading to reactivation of ERK independently of RAS (28). This aberration has been detected in about 20% of melanomas after treatment with BRAF inhibitors. ERK reactivation could be blocked with higher doses of BRAF inhibitors or with the combination of BRAF and MEK inhibitors. However, BRAF amplification also has been detected in patients treated with the combination of MEK and BRAF inhibitors. BRAF splicing is present in 32% of melanomas (29). The combination of BRAF and MEK or single therapy with ERK inhibitors should prevent this phenomenon, although BRAF splicing has been also detected in patients treated with the combination of BRAF and MEK inhibitors.
NRAS mutations
NRAS mutations (Q61, Q12, Q13) occurring al either codon 12 or 61, and with mutations of NF1 drive MAPK activation in 30% of melanomas. BRAF and NRAS mutations are considered to be mutually exclusive. NRAS mutations not only activate MAPK pathway, is thought activate the PI3K pathway.
NRAS mutations are the second most common oncogenic alteration in melanoma (20%) and represent a clinical problem since they are associated with more aggressive tumors and shorter survival in early and late stage melanoma (30,31). The mutation of NRAS, actives transduction signals through CRAF in patients treated with BRAF inhibitors, resulting in a paradoxical transactivation of MAPK signaling via dimerization of BRAF and CRAF (32). Preclinical data in NRAS mutated patients supported the use of MEK, ERK and Pan-RAF inhibitors due to their high level of activity. In clinical trials, a MEK inhibitor (binimetinib) achieved 20% of response rate in NRAS mutant melanoma (33). Two trials have now completed enrollment, one phase II comparing pimasertib versus dacarbacine, and another phase III comparing a MEK inhibitor (binimetinib) versus dacarbacine in an NRAS mutated population. Data presented, but not published of this phase III of binimetinib, showed significant benefit in PFS. Preclinical data are interesting, although the benefit from MEK inhibitors is transient (34). Therefore, one possibility is to look for the last effector of this pathway, in this case blocking CDK4. Clinical trials are ongoing with the combination of MEK and CDK4-6 inhibitors (35). One clinical trial of MEK inhibitor with CDK4/6 inhibitor, binimetinib with LEE011 combination, showed 33% response rate in NRAS mutant population, with good tolerability.
Hyperactivation of RTKS
Overexpression or hyperactivation of RTKs could drive resistance by activation of parallel pathways or by direct induction of the RAS pathway (36). The most frequently involved receptors are platelet derived grow factor receptor beta (PDGFRβ) and insulin-like grow factor I receptor (IGF-1R) (37,38). The activation of these receptors is due to epigenetic changes. The activation of RTKs induces additional activation of the PI3K pathway in patients treated with BRAF or MEK inhibitors, therefore leading to resistance (39). The epidermal growth factor receptor (EGFR) gene is not normally expressed in non-treated melanoma, but in some patients that develop resistance to BRAF or MEK inhibitors overexpression of EGFR is induced by negative feedback. In this case, it is possible restoring sensitivity by inhibiting EGFR (40).
Aberrations in PI3K-PTEN-AKT pathway
MAPK pathway is deregulated in more than 70% of melanomas, but the PI3K/AKT/mTOR pathway is deregulated in more than 50% of melanomas.
It has been found that in 10–20% of cases that develop early resistance, or are intrinsically resistant to the MAPK inhibition, there is a loss of PTEN, or mutations in PI3K or AKT. Experiments in melanoma cell lines supports combined treatment with BRAF/MEK plus PI3K/AKT inhibitors to overcome resistance. Although the results in preclinical models are promising, there is currently limited clinical data (41,42).
Targeted therapy in non-cutaneous melanoma
Uveal melanoma (5% of all melanomas) has mutations in GNAQ/GNA11 (codon 209 or 183) in more than 80% of cases, and result in partial or complete loss of GTPase activity, thereby leading to constitutive activation effector pathways. This aberration activates the MAPK or PI3K pathways or protein kinase C. This activation can be suppressed by PKC inhibitors In a phase II clinical trial, a MEK inhibitor (selumetinib) as monotherapy was compared with chemotherapy. . The study showed a benefit in terms of response rate and PFS, but there was no improvement in terms of OS (43). There are ongoing clinical trials testing the combination of a MEK inhibitor (trametinib) with an AKT inhibitor (GSK2141795), the PKC inhibitor AEB071 as single agent or the combination of combined MEK or PI3K inhibitors.
Mucosal or acral melanomas (3% of all melanomas) harbor mutations or amplifications in cKIT (20–30% of these melanomas) Activating KIT mutations lead to activation of KIT tyrosine kinase activity, stimulate the MAPK and PI3K/AKT pathway; and mutation non amplification predict response to TK inhibitors. The cKIT inhibitor imatinib was tested in in three clinical trials, demonstrating a response rate around 30%. Clinical trials with other ckit inhibitors (nilotinib, dasatinib, sunitinib) have been completed and results are pending (44,45) (Table 2).

Full table
Strategies to overcome resistance
Currently, the combination of BRAF and MEK inhibitors represents the gold standard of targeted therapy in BRAF mutated melanoma. However, even with this combination, efficacy is limited due to development of resistance.
There are several strategies for overcoming such resistance, as combination with other targeted therapies, sequential/intermittent treatment schedules, and the combination of this targeted therapy and with immunotherapy.
The addition of a third drug might help to overcome resistance and several trials are ongoing testing the triple combination of MEK plus BRAF inhibitors with MET, FGF, CDK, VEGF, or mTOR inhibitors (46,47).
It has been demonstrated that in resistant melanoma cell lines harboring BRAF splicing forms or BRAF amplifications stopping BRAF inhibition, leads to melanoma regression (48,49).
Treatment with MAPK inhibitors increases the expression of melanocytic antigens, and CD8 lymphocyte infiltration. This observation supports a possible synergism with the combination of targeted therapy with immunotherapy (50). An early attempt, combining a BRAF inhibitor with anti-CTL4 antibody (ipilimumab), was failed due a high grade of hepatoxicity in the phase I trial that led to an early stop of the study (51). Results of the clinical trial testing the sequential combination of dabrafenib plus ipilimumab are pending. New immunotherapeutic agents, such as anti-PD-1 antibodies (pembrolizumab, nivolumab) demonstrated much higher activity and less toxicity than anti-CTL4 antibody.
The tumor infiltrating cytotoxic CD8 lymphocyte is a component of the adaptive immune response against melanoma associated antigens (after treatment with BRAF inhibitors), circulating CD8 cells sustain a strong inflammatory response with cytotoxic effects. In the exhaustion profile of CD8, leading to their incapacity to proliferate and produce cytokines (IL-2, INF), is mediated up-regulation of inhibitory signaling pathways as PD-1, PD-L1 and CTL4 (52,53). Clinical trials are underway to determine the clinical activity of the combination of BRAF inhibitors with anti PD-1 antibodies (Figure 2).
Preclinical studies have demonstrated that intermittent as opposed to continuous therapy with a BRAF/MEK inhibitor, may delay the development of acquire resistance (54). There are several studies assessing sequential or intermittent dosing of BRAF and MEK inhibitors are ongoing. In the phase II COMBAT study (CT.gov: NCT02224781), patients are randomized to the combination of dabrafenib and trametinib versus their combination, after 8 weeks of monotherapy with dabrafenib or trametinib. Serial biopsies on treatment and at progression are used, to assess biomarkers related to response or resistance. Another clinical trial, SWOG study S1320 (CT.gov: NCT02196181) is looking at intermittent schedule, with the combination with dabrafenib y trametinib during an 8-week lead in period; the patients without disease progression in the lead period, ongoing continuous dosing or to intermittent dosing during 5 weeks on with 3 weeks off. In this study includes serial biopsies to determinate resistance mechanisms.
Reactivation of MAPKinase pathway leds to a highly recurrent transcriptomic alterations across resistant tumors, in contrast to mutations, and were correlated with differential methylation. The authors identified in the tumor: c-MET up-expression, LEF1 down-expression and YAP1 signature enrichment, as a drivers of acquired resistance. The authors observed high intra-tumoral cytolytic T cell inflammation, prior to BRAF inhibitor therapy preceded CD8 T cell exhaustion, and loss of antigen presentation in half of progressive melanomas, suggesting resistance to anti PD-1/PD-L1.
In the presence of BRAF/MEK inhibitor are the adaptive mechanisms of resistance. During the early phase, when the patients still respond to drug with inhibition MAPK pathway, adaptive resistance to BRAF inhibitors can occur, within the first 24−48 hours, leading to dampening of the inhibitor effect. Adaptive signaling seen involves: acquired EGFR and PDGFR expression, increase sensitivity to grow factors as EGF, FGF, HGF, neuregulin-1; increased phosphorylation AKT, up-regulation ERBB3 and enhanced MITF expression (55).
Recently, it has been published that an oncogene MITF is a driver of an early non-mutational and reversible drug tolerance state, which is induced by PAX-3-mediated up-regulation of MITF, before acquire resistance. Nelfinavir, HIV-1 protease inhibitor, was showed as a potent suppressor of PAX3 and MITF expression. Nelfinavir sensitizes BRAF, NRAS and PTEN mutant melanoma cells to MAKP inhibitors (56).
Conclusions
Targeted therapies are highly active drugs against metastatic melanoma. Different mechanisms of resistance have been described: epigenetic (57), genomic (58) and phenotypic (59) changes produces several alterations, leading to intrinsic, acquired or adaptive resistance. Tumor heterogeneity is a major driver of resistance in melanoma. In clinical practice, combination of BRAF and MEK inhibitors is the gold standard for metastatic BRAF mutant melanoma patients. The combination is highly active, but the duration of response is limited due to the development of acquired and adaptive resistance mechanisms. In order to overcome this phenomenon, there are different strategies, as the combination with other drugs—as CDK, PI3K, ERK and AKT inhibitors, intermittent schedules, and the combination with immunotherapy drugs.
Acknowledgements
José Luís Manzano was supported by Fondo de Investigación Sanitaria (FIS)—Instituto de Salud Carlos III (ISCIII). Anna Martínez Cardús was supported by Red Temática de Investigación Cooperativa en Cáncer (RTICC) and Olga Torres Private Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med 2004;351:998-1012. [Crossref] [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [Crossref] [PubMed]
- Menzies AM, Haydu LE, Visintin L, et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res 2012;18:3242-9. [Crossref] [PubMed]
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707-14. [Crossref] [PubMed]
- Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell 2012;150:251-63. [Crossref] [PubMed]
- Sullivan RJ, Flaherty K. MAP kinase signaling and inhibition in melanoma. Oncogene 2013;32:2373-9. [Crossref] [PubMed]
- Curtin JA, Busam K, Pinkel D, et al. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 2006;24:4340-6. [Crossref] [PubMed]
- Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009;457:599-602. [Crossref] [PubMed]
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med 2010;363:2191-9. [Crossref] [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [Crossref] [PubMed]
- Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867-76. [Crossref] [PubMed]
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877-88. [Crossref] [PubMed]
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9. [Crossref] [PubMed]
- Dummer R, Flaherty KT. Resistance patterns with tyrosine kinase inhibitors in melanoma: new insights. Curr Opin Oncol 2012;24:150-4. [Crossref] [PubMed]
- Paraiso KH, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res 2011;71:2750-60. [Crossref] [PubMed]
- Xing F, Persaud Y, Pratilas CA, et al. Concurrent loss of the PTEN and RB1 tumor suppressors attenuates RAF dependence in melanomas harboring (V600E)BRAF. Oncogene 2012;31:446-57. [Crossref] [PubMed]
- Nathanson KL, Martin AM, Wubbenhorst B, et al. Tumor genetic analyses of patients with metastatic melanoma treated with the BRAF inhibitor dabrafenib (GSK2118436). Clin Cancer Res 2013;19:4868-78. [Crossref] [PubMed]
- Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res 2010;70:6670-81. [Crossref] [PubMed]
- Trunzer K, Pavlick AC, Schuchter L, et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol 2013;31:1767-74. [Crossref] [PubMed]
- Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014;4:80-93. [Crossref] [PubMed]
- Smalley KS, Lioni M, Dalla Palma M, et al. Increased cyclin D1 expression can mediate BRAF inhibitor resistance in BRAF V600E-mutated melanomas. Mol Cancer Ther 2008;7:2876-83. [Crossref] [PubMed]
- Flaherty KT, Lorusso PM, Demichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res 2012;18:568-76. [Crossref] [PubMed]
- Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012;487:500-4. [Crossref] [PubMed]
- Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012;487:505-9. [Crossref] [PubMed]
- Gibney GT, Smalley KS. An unholy alliance: cooperation between BRAF and NF1 in melanoma development and BRAF inhibitor resistance. Cancer Discov 2013;3:260-3. [Crossref] [PubMed]
- Krauthammer M, Kong Y, Ha BH, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 2012;44:1006-14. [Crossref] [PubMed]
- Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov 2014;4:94-109. [Crossref] [PubMed]
- Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 2011;480:387-90. [Crossref] [PubMed]
- Shi H, Moriceau G, Kong X, et al. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun 2012;3:724. [Crossref] [PubMed]
- Devitt B, Liu W, Salemi R, et al. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment Cell Melanoma Res 2011;24:666-72. [Crossref] [PubMed]
- Jakob JA, Bassett RL Jr, Ng CS, et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2012;118:4014-23. [Crossref] [PubMed]
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 2010;140:209-21. [Crossref] [PubMed]
- Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013;14:249-56. [Crossref] [PubMed]
- Kwong LN, Costello JC, Liu H, et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat Med 2012;18:1503-10. [Crossref] [PubMed]
- Sosman JA, Kittaneh M, Lokelma MPJK, et al. A phase 1b/2 study of LEE011 in combination with binimetinb in patients NRAS mutante melanoma: early encouraging clinical activity. J Clin Oncol 2014;32:abstr 9009.
- Deng W, Gopal YN, Scott A, et al. Role and therapeutic potential of PI3K-mTOR signaling in de novo resistance to BRAF inhibition. Pigment Cell Melanoma Res 2012;25:248-58. [Crossref] [PubMed]
- Gopal YN, Deng W, Woodman SE, et al. Basal and treatment-induced activation of AKT mediates resistance to cell death by AZD6244 (ARRY-142886) in Braf-mutant human cutaneous melanoma cells. Cancer Res 2010;70:8736-47. [Crossref] [PubMed]
- Shi H, Kong X, Ribas A, et al. Combinatorial treatments that overcome PDGFRβ-driven resistance of melanoma cells to V600EB-RAF inhibition. Cancer Res 2011;71:5067-74. [Crossref] [PubMed]
- Goel VK, Lazar AJ, Warneke CL, et al. Examination of mutations in BRAF, NRAS, and PTEN in primary cutaneous melanoma. J Invest Dermatol 2006;126:154-60. [Crossref] [PubMed]
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature 2014;508:118-22. [Crossref] [PubMed]
- Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov 2011;1:248-59. [Crossref] [PubMed]
- Chandarlapaty S, Sawai A, Scaltriti M, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 2011;19:58-71. [Crossref] [PubMed]
- Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA 2014;311:2397-405. [Crossref] [PubMed]
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol 2013;31:3182-90. [Crossref] [PubMed]
- Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011;305:2327-34. [Crossref] [PubMed]
- Lee B, Sandhu S, McArthur G. Cell cycle control as a promising target in melanoma. Curr Opin Oncol 2015;27:141-50. [Crossref] [PubMed]
- Sullivan RJ, Fisher DE. Understanding the biology of melanoma and therapeutic implications. Hematol Oncol Clin North Am 2014;28:437-53. [Crossref] [PubMed]
- Das Thakur M, Salangsang F, Landman AS, et al. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013;494:251-5. [Crossref] [PubMed]
- Das Thakur M, Stuart DD. Molecular pathways: response and resistance to BRAF and MEK inhibitors in BRAF(V600E) tumors. Clin Cancer Res 2014;20:1074-80. [Crossref] [PubMed]
- Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013;19:1225-31. [Crossref] [PubMed]
- Sullivan RJ, Lorusso PM, Flaherty KT. The intersection of immune-directed and molecularly targeted therapy in advanced melanoma: where we have been, are, and will be. Clin Cancer Res 2013;19:5283-91. [Crossref] [PubMed]
- Jiang X, Zhou J, Giobbie-Hurder A, et al. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res 2013;19:598-609. [Crossref] [PubMed]
- Atefi M, Avramis E, Lassen A, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res 2014;20:3446-57. [Crossref] [PubMed]
- Das Thakur M, Stuart DD. The evolution of melanoma resistance reveals therapeutic opportunities. Cancer Res 2013;73:6106-10. [Crossref] [PubMed]
- Hugo W, Shi H, Sun L, et al. Non-genomic and Immune Evolution of Melanoma Acquiring MAPKi Resistance. Cell 2015;162:1271-85. [Crossref] [PubMed]
- Smith MP, Brunton H, Rowling EJ, et al. Inhibiting Drivers of Non-mutational Drug Tolerance Is a Salvage Strategy for Targeted Melanoma Therapy. Cancer Cell 2016;29:270-84. [Crossref] [PubMed]
- Vizoso M, Ferreira HJ, Lopez-Serra P, et al. Epigenetic activation of a cryptic TBC1D16 transcript enhances melanoma progression by targeting EGFR. Nat Med 2015;21:741-50. [Crossref] [PubMed]
- Lin L, Sabnis AJ, Chan E, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet 2015;47:250-6. [Crossref] [PubMed]
- Roesch A. Tumor heterogeneity and plasticity as elusive drivers for resistance to MAPK pathway inhibition in melanoma. Oncogene 2015;34:2951-7. [Crossref] [PubMed]



