The emerging role of immunotherapy in head and neck squamous cell carcinoma (HNSCC): anti-tumor immunity and clinical applications
Introduction
Head and neck squamous cell carcinoma (HNSCC) represents a heterogeneous disease entity, which encompasses a variety of tumors originating in the lip/oral cavity, hypopharynx, oropharynx, nasopharynx or larynx with differences in epidemiology, etiology and therapeutical approach. It is the sixth most common malignancy worldwide, accounting for approximately 6% of all cases and is responsible for an estimated 1–2% of all cancer deaths (1).
HNSCC has been historically associated with tobacco and alcohol use; however, in the past decade, infection with high-risk human papillomaviruses (HPV) and especially type 16 has been implicated in the pathogenesis of a subset of HNSCCs, mainly those arising from the oropharynx. HPV-associated oropharyngeal cancer represents a distinct biological and clinical entity with a more favorable prognosis (2,3). This raises the question whether HPV positive patients should be treated with less intensive treatment, which is currently being addressed in clinical trials. The majority of HNSCC patients present with locally advanced disease for which multimodality therapeutic approach is employed. For recurrent/metastatic (R/M) disease, cytotoxic-based chemotherapy remains the standard therapeutic option and the median survival of patients treated with palliative chemotherapy alone ranges from 6 to 10 months (4).
Low survival rates in combination with significant toxicities caused by current treatment strategies used in HNSCC underlines the urgent need for enhanced treatment options. It has been widely accepted that the immune system plays a crucial role in cancer development, as tumor cells evade immunosurveillance by exploiting inhibitory checkpoint pathways that suppress antitumor T-cell responses (5). HNSCC has been intensely studied as an immunosuppressive disease. Following the increasing understanding of the underlying mechanisms behind control of malignancies by the immune system, the establishment of immune-based therapies has emerged as a promising approach for the treatment of cancer.
In this review, we will focus on the role of immune system in HNSCC tumorigenesis and describe immunotherapy approaches currently under investigation.
Immune system and cancer development
Cancer is a multistep process originating from genetic alterations in normal proliferation and differentiation. In a normally functioning environment, immune surveillance acts as an effective tumor suppressor mechanism, as these alterations trigger the development of tumor-related antigens initially recognized by the immune system. However, it is believed that after this equilibrium phase, immune system might lose the ability to eradicate cancer cells, or new mutations might render tumor cells poorly immunogenic and resistant to elimination by immune cells (6-8).
Both the innate and adaptive immune systems have the ability to distinguish between self and non-self pathogens. Innate immunity is based on non specific defense mechanisms that are activated immediately after contact with pathogen; it uses a limited number of receptors that are encoded in the germline and are able to recognize features common to many pathogens. In contrast, adaptive immunity relies upon somatic cell gene rearrangements to produce a multitude of antigen receptors that discriminate between closely related molecules; it is mainly driven by highly specific antigen receptors on T and B cells and is highly specific to a particular pathogen (9).
The immune system can identify and eliminate tumor cells based on their expression of tumor-associated antigens (ΤΑΑ) via a process termed immunosurveillance. Tumors can express microbial proteins, mutated proteins, and fusion proteins. The immune system can also recognize aberrantly expressed self proteins (10). During tumor evolution, T-cells are activated upon encounters with antigen-presenting cells (APC), usually dendritic cells (DC), B-cells or macrophages that display TAA, which are bound to major histo-compatibility complex proteins (MHC) and interact with T cell receptors (TCRs). A complex network of co-stimulatory and co-inhibitory pathways that normally play a pivotal role in the prevention of auto-immunity, are manipulated by cancer cells to escape immunosurveillance. Co-stimulatory or activating receptors include CD28, CD137, CD40, and OX-40 (11). Upon secretion of specific chemokines, a proportion of T-cells differentiate into cytotoxic CD8+ cells that move to the tumor microenvironment and directly attack tumor cells. Using gene expression profiling, studies initially conducted in malignant melanoma have led to identification and description of two separate subtypes of tumor microenvironment based on the presence of a transcriptional profile denotative of T cell infiltration (12). More specifically, “inflamed” tumor immunophenotype is characterized by recruitment of T-cells, immune signals and chemokines, whereas “non-inflamed” tumor phenotype lacks spontaneous infiltration of T-cells and other immunomodulators. Most importantly, it is suggested that in the subset of patients sharing the “inflamed phenotype” tumor progression might be a result of negative immune regulators acting at the level of the microenvironment. On the contrary, failure in “non-inflamed” tumors is attributed to poor effector T cell trafficking at the site of the tumor (13,14).
The role of immune system in head and neck cancer development and progression
Emerging evidence supports a vital role of the immune system in the development and evolution of HNSCC. Furthermore, the status of the immune system is likely to be of prognostic value in HNSCC. HNSCC is considered an immunosuppressive disease, characterized by dysregulation of immunocompetent cells and impaired cytokine excretion (15). Immunosuppressive individuals are prone to develop head and neck cancer and prognosis is poor (16). For HPV(–) HNSCC, although there is a strong causative association with tobacco and alcohol, it is hypothesized that tumor progression reflects the inability of the immune system to eliminate the cancer. As various cells of the immune system provide a complex network of defense, the balance between subsets of T-lymphocytes, combined with the effect of tumor microenvironment, are believed to modulate antitumor immunity (17). T-cells, macrophages, dendritic and natural killers (NK) cells are important players in the tumor microenvironment, whose functional alterations modify immune response.
Patients with HNSCC have reduced antitumor immune responses, and tumor progression or relapse is believed to be associated with immune dysfunction. Several mechanisms, such as the presence of tumor-secreted proteins that act as inhibitory stimuli, cytokines and T cell apoptosis have been suggested to contribute to immune deregulation (18). Importantly, the presence of T-regulatory cells (Tregs) has emerged as a potential mechanism of immunomodulation in HNSCC. Tregs suppress or down-regulate induction and proliferation of effector T cells and have been found to be consistently observed at a high frequency in patients with HNSCC (19,20). Surprisingly, unlike other solid malignancies including lung cancer and renal cell cancer, the presence of Tregs has been found to correlate with good clinical outcome (21-23). Multiple functions of Tregs explain this paradox, including suppression of inflammation triggered by immune cells, elimination of macrophages that have a protumor effect in cancer development and induction of apoptosis (24). Recent studies suggest the presence of different subsets of Tregs with functional heterogeneity (25). On the other hand, low levels of CD4+ and CD8+ T cells have been found in patients with HNSCC, particularly those with active disease, suggesting decreased function of effector cells in this population. However, even in patients with no evidence of disease, immune abnormalities may persist after weeks or years of curative therapy (26).
The role of the immune system is also important in HPV-associated OPC. HPV infection is common, but a minority of individuals will develop cancer. Failure of the immune system to clear the oncogenic infection accounts for a minority of cases that finally develop cancer; persistence of HPV infection in lymphoid tissue of the head and neck might be related to self-regulatory mechanisms that allow these tissues to sample the oral environment without continuous immune activation (16). Following establishment of HPV infection, HPV specific effector T cells are responsible for elimination of the virus and HPV-induced oncogenesis has been shown to correlate with weak HPV-specific T cell responses (27). On the other hand, PD1, a protein functioning as immune checkpoint by preventing the activation of T-cells, has been found in tonsilar crypts and PD1 infiltrating lymphocytes have been identified in HPV(+)-OPC, PD1 pathway might play a key role in HPV-related OPC oncogenesis (28). Interestingly, PD-1 positive infiltrating cells have been associated with more favorable clinical outcome (29,30).
To date, only few studies have examined the prognostic impact of tumor infiltrating lymphocytes (TILs) in correlation with HPV status in HNSCC patients. Several studies have demonstrated an increased population of CD8+ lymphocytes, Tregs and increased CD8+/Treg ratio in HPV+ OPC, which are associated with improved prognosis (31,32).
The cancer stem cell hypothesis supports the notion that in a heterogenic tumor, a subpopulation of tumor cells (cancer stem cells-CSCs) is capable of initiating and expanding a tumor (33). In HNSCC, it has been proposed that slow growing CSCs evade conventional therapies and regenerate the tumor, accounting for the high rates of recurrence (34). Emerging evidence suggests that host immune system has the ability to recognize CSCs and provoke an immune response. Early studies in HNSCC have shown that NK cells may preferably target CSCs (35). In addition, CSCs interact with tumor microenvironment. In HPV-related HNSCC, the presence of CSCs is controversial (36-38). Interestingly, CSCs have been associated with radioresistance and cisplatin-resistance in HNSCC (39,40).
Although immunoediting may eliminate tumor cells with alterations in their antigenic epitope profile, many immunoresistant variants escape from the immune system of the host by immunosuppressive molecular and cellular mechanisms. Therefore, tumors may avoid elimination by the immune system through outgrowth of tumor cells that can suppress, disrupt, or escape the immune system.
In HNSCC, the dominant mechanism of immune-escape is inhibition of tumor antigen presentation. Disruption of antigen presentation can occur by (I) down-regulation or loss of tumor human leukocyte antigen (HLA) class I molecules expression; (II) disruption of proteins involved in antigen processing, such as TAP1, LMP2, LMP7; and (III) suppression of APC function and maturation (10,41). Large scale next generation sequencing of HNSCC has revealed several mutations in HLA alleles and APM components, but tumor cells avoid complete loss of HLA expression, as it leads to recognition by NK cells (42). It has been also proposed that signal transducer and activator of transcription (STAT) family of proteins controls defects in APM, as well as APC function. For instance, deficiency of activated STAT1 results in reduced expression of APM components, while aberrant signaling of STAT3 contributes to impaired tumor antigen presentation by DCs (43,44). Nevertheless, disruption of TAA presentation results in functionally defective circulating lymphocytes that have enhanced levels of apoptosis and increased suppression induced by Tregs (45).
Second, the tumor microenvironment contains various immunosuppressive factors from different sources that tumors use as defense mechanisms against the immune system. HNSCC microenvironment is characterized by a deranged cytokine profile, promoting the secretion of immunosuppressive over stimulating cytokines. VEGF, an inflammatory cytokine released from tumor cells, inhibits DC function; it has been found in high concentrations in patients with HNSCC and has been correlated with relapse (46,47). Upregulation of IL-6 activates the STAT3 pathway, which subsequently inhibits DC maturation and NK, T and macrophage activation. Overexpression of STAT3 also increases the production of IL-10 and TGF-b (48). TGF-β has suppressive effects on effector cells and APCs, whereas IL-10 downregulates expression of co-stimulatory molecules and MHC (49). Furthermore, overexpression of diverse chemokines by the HNSCC tumor cells mediate the recruitment of suppressive myeloid cells, such as myeloid-derived suppressive cells (MDSC) and tumor-associated macrophages (TAMs); deregulation of immune cell recruitment limits the anti-tumor immune response (42,50).
Recruitment of immunosuppressive cells in tumor microenvironment plays a major role in immune escape in HNSCC. MDSCs are immature myeloid populations that are recruited to the tumors after secretion of soluble immunosuppressive factors, such as PGE2, IL-6, GM-CSF, IL-10, VEGF, and TGF-b1 (51). They cause repression of T-cell activation in HNSCC (42). Following cytokine release, arginase-1 enzyme is produced that metabolizes L-arginine, activate iNOS, and controls the tumor release of indoleamine-2,2-dioxygenase (IDO), which catabolizes the essential for the differentiation of T-cell amino acid tryptophan, leading to the immunosuppression of T-cell response. On the other hand, TAMs have been identified as key regulators of tumor immunosuppression, migration and metastasis. Phenotypically, macrophages can be either M1 or M2. M1 macrophages contribute to immune response against malignant cells by activating Th1 cells, whereas M2 macrophages produce an anti-inflammatory response via cytokine release that promotes tumor growth (52). In HNSCC, TAMs are associated with worse outcome post-surgery and chemoradiotherapy (53,54). Finally, Tregs, often expressed in patients with HNSCC, can suppress immune responses via production of IL-10 and TGF-β, using up environmental T-cell survival factors, and dysregulating local T cells (55).
On the other hand, tumors can act through a variety of mechanisms, some of which are not well understood, to inhibit or prevent attack by immune cells. The most characteristic example is through expression of PD-L1. Immune checkpoints, such as PD-1 pathway, are part of a protein-ligand receptor system that controls T-cell activation. They are critical for protecting self-tolerance and modulating the duration and amplitude of physiologic immune response, but are manipulated by cancer to permit tumor growth that is unchecked by the immune system. In a normal cell, when PD-L1 or PD-L2 binds to PD-1, the T cell becomes inactive. This is a way that the body regulates the immune system, to avoid an overreaction. However, PD-L1 is also expressed in HNSCC, resulting in disarming of T cells after binding to PD-1 on T cells (56). The concentration of TILs is inversely associated with PD-L1 expression of tumor cells (57). CTLA-4 is another immune checkpoint located on the surface of activated CTLs that binds to the B7 ligands found on APCs. T-cells have a CD28 receptor that represents a stimulatory counterpart to CTLA4, causing T-cell activation. CTLA-4 competes with CD28 receptor for binding to the B7 ligand resulting in either an inhibitory or stimulatory effect on T-cells (11).
Immunotherapeutic strategies for HPV-induced HNSCC
HPV-associated HNSCC represents a subset of OPC patients with unique characteristics that might require less intensive treatment than their tobacco-induced counterparts. Improvement in survival is independent of available conventional treatments and there is a concern of unnecessary toxicity. It has been suggested that clinical trials should discriminate between HPV(+) and HPV(–) patients. HPV-targeted immunotherapy represents a therapeutic approach that might allow clinicians to use conventional treatment at lower doses, reducing treatment-related toxicity. Viral oncoproteins E6 and E7 represent good targets for immunotherapy, as they are continuously expressed by tumor cells and are essential to maintain the transformation status of HPV+ oropharyngeal cancer cells (2).
The primary goal of prophylactic vaccination is to induce an immune response such that high-titers of HPV-neutralizing antibodies are produced that are capable of preventing initial infections, making HPV antigen-specific B cells the target cell type for these vaccines. On the contrary, therapeutic vaccines focus on the generation of CD8+ HPV-specific T cell immune response. E6 and E7 oncoproteins are most frequently targeted for vaccine development (58).
Vaccine mediated immune strategies are either prophylactic against primary infection with the view to prevent carcinogenesis or therapeutic in established HPV-associated HNSCC targeting E6 and E7 oncoproteins. HPV preventive vaccines are based on virus-like particles assembled from recombinant HPV protein and contain inactive L1 capsid proteins; they act by eliciting virus-neutralizing antibody responses that prevent initial infection (59). Gardasil (Merck) and Cervarix (GlaxoSmithKline) are two commercially available HPV vaccines shown to be effective for cervical carcinoma in large randomized trials (60). Their role in prevention of HPV-related oropharyngeal cancers is currently being evaluated, with one trial showing promising results (61). A trial testing efficacy of Gardasil in 11-year-old boys in Mexico City is underway (NCT02382900). Of note, due to loss of L1 expression after established HPV infection, preventive vaccines are not effective in HPV-related HNSCC.
Several vaccination therapies are under investigation in HPV-associated HNSCC. DNA vaccines produce non-living antigens able to induce CTL, Th and B cell immunity. Their benefits include safety, low cost and easy production (62). Multiple DNA vaccine trials targeting HPV are being tested in cervical cancer. A phase I trial is currently assessing safety and feasibility of administration of pNGVL4a-CRT/E7 (Detox) DNA Vaccine in combination with cyclophosphamide in HPV+ OPC (NCT01493154). Vaccine pNGVL4a-CRT/E7 consists of the DNA plasmid pNGVL4a-A encoding calreticulin, linked to a detox form of human papillomavirus (HPV) type 16 E7 antigen. On the other hand, peptide vaccines incorporate amino acid sequences that are synthesized to form an immunogenic peptide molecule representing the specific epitope of a TTA that binds onto HLA. After activation of CTLs by the peptide vaccine, cells can recognize peptide-MHC I complex on tumor cells (63). Several peptide vaccines are under evaluation in HPV+ HNSCC. In a phase I trial, five patients with advanced HNSCC were treated with peptide vaccines composed of HLA-I and HLA-II restricted melanoma antigen E (MAGE)-A3 or HPV-16 derived peptides, provoking a measurable immune response and acceptable toxicity (64). Furthermore, a phase II trial evaluating the efficacy of HPV16 E6 and E7 peptide vaccines in patients with HPV-related tumors including HNSCC has been completed and results are expected shortly (NCT00019110).
Vaccination strategies involving DCs are currently being assessed in HPV+ HNSCC. DC vaccines are produced by culturing ex vivo DCs that have been derived from patients with the HPV antigen; after maturation and activation, DCs cells are injected back into the patient (42). A phase I trial testing the safety and efficacy of intratumoral injection of a DC vaccine in patients with advanced HNSCC has been unfortunately withdrawn (NCT00492947). On the other hand, several bacterial HPV vaccines targeting E6 and E7 have been developed. Sewell et al. showed that in an HPV 16 transfected mouse model, mice that were vaccinated with Listeria based anti E7 vaccine experienced a substantial reduction in tumor size (65). Interestingly, an ongoing clinical study evaluates the efficacy of neoadjuvant listeria-based HPV vaccine ADX11-001 in patients with HPV+ HNSCC stage I–IV undergoing robot-assisted surgery (NCT02002182).
Finally, adoptive T-cell transfer (ACT) might be a promising immunotherapy strategy for HPV HNSCC; it involves harvesting and ex vivo expansion of the patient’s own tumor antigen specific T-cells. Subsequently, T-cells are re-introduced to the patient, with the view to enhance immunity and improve anticancer immune response (66). An ongoing phase II clinical trial is assessing the efficacy of lymphodepletion followed by autologous infusion of TILs in patients with HPV+ advanced solid tumors including OPC (NCT01585428).
Immunotherapeutic strategies for HPV(–) HNSCC
Improved understanding of the role of the immune system in cancer has led to the identification of a range of novel therapeutic targets. Immuno-oncology is an evolving field of investigation that includes active immunotherapies that are designed to target and harness the patient’s own immune system directly to fight cancer. More specifically, it is designed to leverage the unique properties of the immune system (specificity, adaptability, and memory).The primary goal of immunotherapy is to shift the balance in favor of an immune response against the tumor, allowing tumor eradication or long-term suppression of tumor growth, and the generation of immunological memory.
Monoclonal antibodies
Cetuximab is a chimeric immunoglobulin G1 (IgG1) monoclonal antibody that has been approved by the US Food and Drug Administration (FDA) in combination with chemotherapy as the standard first line treatment for R/M HNSCC. It is also used in conjunction with radiation for locally advanced HNSCC (67,68). Cetuximab efficacy is mediated by antibody-dependent cell mediated cytotoxicity (ADCC), a mechanism of cell-mediated immune defense whereby NK cells, actively lyse a target cell, whose membrane-surface antigen has been bound by cetuximab. NK cells are activated upon binding to surface receptor FCγRIIIa (69). Furthermore, cetuximab provokes CTL antitumor response through cross-priming of DCs and NKs (46). Other anti-EGFR monoclonal antibodies currently evaluated in HNSCC include panitumumab, nimotuzumab and zalutumumab. Among them, panitumumab has produced modest results when added to platinum based chemotherapy in patients with R/M HNSCC (70). Zalutumumab has demonstrated an OS of 5.3 months and a PFS of 2.1 when administered as monotherapy in patients with platinum refractory R/M HNSCC (71). Finally, nimotuzumab in combination with (chemo) radiation in locally advanced HNSCC has shown a survival benefit in tumors overexpressing EGFR (72).
Immune checkpoint inhibitors
It is now clear that tumors co-opt certain immune-checkpoint pathways as a major mechanism of immune resistance, particularly against T cells that are specific for tumor antigens. Because many of the immune checkpoints are initiated by ligand-receptor interactions, they can be readily blocked by antibodies or modulated by recombinant forms of ligands or receptors. Ipilimumab, a mAb against CTLA-4 that has received FDA approval for metastatic melanoma, is currently being evaluated in clinical trials in combination with cetuximab and intensity-modulated radiotherapy (IMRT) in patients with advanced HNSCC (NCT01860430 and NCT01935921). A phase 1, open-label, dose escalation study of MGA271 [enoblituzumab, a humanized mAb against CD276 (B7-H3) in combination with ipilimumab in patients with B7-H3-expressing HNSCC and other solid tumors] is also ongoing (NCT02381314). Tremelimumab is another anti-CTLA4 antibody currently being assessed in clinical trials.
PD-1 interacts with two ligands; PD-L1, that is expressed mainly on tumor cells and other immune cells and PD-L2, which is expressed primarily on macrophages and DCs. Anti-PD-1 antibody pembrolizumab (MK-3475) has shown promising results in HNSCC. Preliminary results from KEYNOTE-012, a phase I assessing the efficacy of pembrolizumab in patients with R/M HNSCC had shown a response rate of 20% in PD-L1 positive tumors. Interestingly, 78% were found to be PD-L1-positive. Responses were observed in both HPV+ and HPV– patients, but overall survival was better in HPV+ patients. Response duration ranged from 8 to 41 weeks. PD-L1 expression was positively associated with ORR (P=0.018) and PFS (P=0.024). A larger HNSCC expansion cohort of KEYNOTE-012 was recently presented in the 2015 ASCO meeting, demonstrating an overall response rate (ORR) of 18.2%, whereas 31.3% of patients had stable disease; response rates were similar in HPV+ and HPV– HNSCC. Toxicity was tolerable, with 7.6% of patients experiencing > grade 3 drug-related events (73). Pembrolizumab is further assessed in multiple clinical settings in HNSCC. In KEYNOTE-048, it is evaluated either as monotherapy or in combination with chemotherapy versus standard chemotherapy in patients with R/M HNSCC in the first line setting (NCT02358031). In KEYNOTE-040, pembrolizumab is compared to chemotherapy or cetuximab in the second line setting (NCT02252042). Pembrolizumab is also currently evaluated in combination with re-irradiation and as part of primary treatment in various clinical trials (NCT02289209, NCT02296684). Anti-PD-1 Abs nivolumab (NCT02105636; n=340 patients) and pembrolizumab (NCT02358031; n=750 patients) are being evaluated as a single agents in randomized phase III trials for platinum-refractory HNSCC. More specifically, checkmate-141 phase III trial assessed the efficacy of nivolumab versus physician’s choice (cetuximab, methotrexate, or docetaxel) in platinum refractory disease. The study was terminated early after an independent monitoring panel determined the primary endpoint of improvement in OS was met with nivolumab. Another promising anti PD-L1 antibody is durvalumab (MEDI4736), which has shown promising results (∼14% response rate as per RECIST criteria, with 24% response rate in PD-L1+ patients) in a phase I trial (74). A phase III trial evaluating durvalumab alone or in conjunction with tremelimumab compared to standard treatment is under way in patients with advanced HNSCC (NCT02369874).
Another group of receptors with a modulating effect on immune cells includes other checkpoint receptors such as LAG-3 or the killer-cell immunoglobulin-like receptors (KIRs) (75). They regulate immune response via interaction with MHC I molecules. Most of the receptors suppress cytotoxicity, mainly by turning off NK cells when HLA is expressed on tumor cells. Ongoing trials are testing an anti-KIR moAb in combination with ipilimumab (NCT01750580) or nivolumab (NCT01714739). Anti-PD-1 monoclonal antibodies are also being studied in various novel combinations in phase I setting, such as nivolumab plus agonistic anti-CD137 moAbs (urelumab, NCT02253992), nivolumab plus anti-LAG-3 (NCT01968109), and cetuximab plus urelumab.
DC vaccines
DC vaccines have received considerable interest due to their capacity of inducing a robust immunity reaction. As described before, they are manufactured via isolation of DCs and loading of tumor antigen ex vivo, followed by re-introduction of DCs into the patients as a cellular vaccine, usually into the tumor or into lymphnodes. In a preclinical study, a DC vaccine was developed using a skin flap transfer treated with sensitized DCs in a rat tumor model. It was observed that the DC-treated group showed a reduction in tumor size and an immunological response, defined as elevated levels of IL-2 and IFN-γ (76). A phase I trial has been conducted in stage I–IVa patients with HNSCC with no active disease using a DC vaccine loaded with two HLA-A*0201-restricted T cell-defined p53 peptides alone, plus either a wt p53 helper peptide or nonspecific helper peptide derived from tetanus toxoid. In this study, disease-free survival was 88% and p53-specific T cell frequencies were increased in approximately 70% of patients, whereas toxicity was acceptable (77). Finally, in another study, autologous DCs loaded with apoptotic tumor cells were injected intranodally in patients with advanced HNSCC; immunological responses were satisfactory and all patients were long term survivors (78).
Adoptive T cell therapy (ACT)
As previously described, ACT is a therapeutic procedure where T cells are isolated from peripheral blood mononuclear cells of patients or from TILs of primary tumor, undergo in vitro expansion and are re-infused into the patient, with the view to enhance anti-tumor immune response. Genetic engineering of T cells before re-introduction potentially augments function through several autonomous mechanisms. In a phase I study conducted in 17 patients with R/M HNSCC, patients were vaccinated on the thigh with irradiated autologous tumor cells; subsequently, T-cells derived from resected inguinal lymphnodes were expanded in vitro and re-introduced into the patients. Among the patients enrolled, 6/17 patients experienced disease control (79). Importantly, efficacy of ACT is enhanced by cytotoxic chemotherapy. In a retrospective study, ACT was added as experimental therapy in patients with resectable HNSCC receiving induction chemotherapy. Interestingly, median OS and PFs were improved in patients treated with ACT (80). Finally, ACT has been assessed in patients with R/M nasopharyngeal carcinoma. In a phase II study, ACT with EBV-specific CTLs in combination with chemotherapy has shown promising results, demonstrating a 2-year OS of 62.9% (81).
Table 1 summarizes ongoing immunotherapy studies.
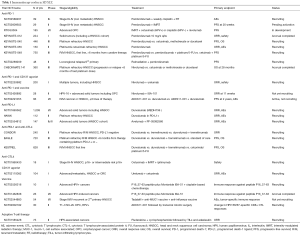
Full table
Combination of immunotherapies
The combination of immunotherapeutic strategies represents a challenging approach, with a view to enhance antitumor immunity by targeting several aspects of immune response. In malignant melanoma, the combination regimen of anti-PD-1 antibody nivolumab and anti-CTL4 antibody ipilimumab has been approved by the FDA after yielding promising results in a phase III trial. A phase III study (KESTREL) is evaluating concurrent tremelimumab and durvalumab vs. durvalumab monotherapy vs. standard of care (EXTREME) as first-line treatment in patients with recurrent and/or metastatic HNSCC. This promising clinical trial design combining two immune checkpoint inhibitors aims to produce deep and durable antitumor responses, which thus far have been observed in only a minority of patients with monotherapy approaches. The phase I, open label, dose-escalation and expansion study evaluating durvalumab and tremelimumab in advanced solid tumors showed a 27% response rate (95% CI, 13–46) in PD-L1 negative patients, with a disease control rate of 48% (95% CI, 31–66) at ≥16 weeks after therapy. Notably, anti-PD1/anti-PD-L1 monotherapy yields an approximately 5–10% response rate in PD-L1 negative patients; therefore, the addition of low-dose anti-CTLA-4 may benefit these patients.
Conclusions and future directions
HNSCC represents a heterogeneous group of diseases. To date, conventional treatment has mediocre results and prognosis in patients with advanced disease is dismal. There is a growing body of evidence that the immune system plays a pivotal role in oncogenesis and tumor evolution; immunoediting is the term used to describe the immune system’s protective role against cancer development. Genetic and epigenetic alterations that are characteristic of all cancers provide a diverse set of antigens that the immune system can use to distinguish tumor cells from their normal counterparts. However, tumors have the capacity to manipulate the immune system in their favor. Our better understanding of the mechanisms of immune escape has led to the development of novel immunotherapies that has shown initial promising results in many solid tumors including HNSCC. A plethora of novel strategies is being explored in clinical trials with the view to improve patient outcome.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Rampias T, Sasaki C, Weinberger P, et al. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J Natl Cancer Inst 2009;101:412-23. [Crossref] [PubMed]
- Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 2006;24:736-47. [Crossref] [PubMed]
- Baxi S, Fury M, Ganly I, et al. Ten years of progress in head and neck cancers. J Natl Compr Canc Netw 2012;10:806-10. [PubMed]
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008;8:467-77. [Crossref] [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004;21:137-48. [Crossref] [PubMed]
- Raval RR, Sharabi AB, Walker AJ, et al. Tumor immunology and cancer immunotherapy: summary of the 2013 SITC primer. J Immunother Cancer 2014;2:14. [Crossref] [PubMed]
- Mendes F, Domingues C, Rodrigues-Santos P, et al. The role of immune system exhaustion on cancer cell escape and anti-tumor immune induction after irradiation. Biochim Biophys Acta 2016;1865:168-75.
- Medzhitov R, Janeway CA Jr. Decoding the patterns of self and nonself by the innate immune system. Science 2002;296:298-300. [Crossref] [PubMed]
- Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011;29:235-71. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Woo SR, Corrales L, Gajewski TF. The STING pathway and the T cell-inflamed tumor microenvironment. Trends Immunol 2015;36:250-6. [Crossref] [PubMed]
- Spranger S, Gajewski T. Rational combinations of immunotherapeutics that target discrete pathways. J Immunother Cancer 2013;1:16. [Crossref] [PubMed]
- Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin Oncol 2015;42:663-71. [Crossref] [PubMed]
- Varilla V, Atienza J, Dasanu CA. Immune alterations and immunotherapy prospects in head and neck cancer. Expert Opin Biol Ther 2013;13:1241-56. [Crossref] [PubMed]
- Schoenfeld JD. Immunity in head and neck cancer. Cancer Immunol Res 2015;3:12-7. [Crossref] [PubMed]
- Chikamatsu K, Sakakura K, Whiteside TL, et al. Relationships between regulatory T cells and CD8+ effector populations in patients with squamous cell carcinoma of the head and neck. Head Neck 2007;29:120-7. [Crossref] [PubMed]
- Whiteside TL. Down-regulation of zeta-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother 2004;53:865-78. [Crossref] [PubMed]
- Reichert TE, Strauss L, Wagner EM, et al. Signaling abnormalities, apoptosis, and reduced proliferation of circulating and tumor-infiltrating lymphocytes in patients with oral carcinoma. Clin Cancer Res 2002;8:3137-45. [PubMed]
- Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res 2002;8:2553-62. [PubMed]
- Siddiqui SA, Frigola X, Bonne-Annee S, et al. Tumor-infiltrating Foxp3-CD4+CD25+ T cells predict poor survival in renal cell carcinoma. Clin Cancer Res 2007;13:2075-81. [Crossref] [PubMed]
- Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res 2006;12:465-72. [Crossref] [PubMed]
- Tao H, Mimura Y, Aoe K, et al. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer 2012;75:95-101. [Crossref] [PubMed]
- Badoual C, Hans S, Fridman WH, et al. Revisiting the prognostic value of regulatory T cells in patients with cancer. J Clin Oncol 2009;27:e5-6; author reply e7.
- Sun W, Li WJ, Wu CY, et al. CD45RA-Foxp3high but not CD45RA+Foxp3low suppressive T regulatory cells increased in the peripheral circulation of patients with head and neck squamous cell carcinoma and correlated with tumor progression. J Exp Clin Cancer Res 2014;33:35. [Crossref] [PubMed]
- Schaefer C, Kim GG, Albers A, et al. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer 2005;92:913-20. [Crossref] [PubMed]
- van der Burg SH. Immunotherapy of human papilloma virus induced disease. Open Virol J 2012;6:257-63. [Crossref] [PubMed]
- Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 2013;73:1733-41. [Crossref] [PubMed]
- Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res 2013;73:128-38. [Crossref] [PubMed]
- Vasilakopoulou M, Rampias T, Sasaki C, et al. Effect of PDL-1 expression on prognosis in head and neck squamous cell carcinoma. J Clin Oncol 2013;31:abstr 6012.
- Näsman A, Romanitan M, Nordfors C, et al. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS One 2012;7:e38711. [Crossref] [PubMed]
- Lukesova E, Boucek J, Rotnaglova E, et al. High level of Tregs is a positive prognostic marker in patients with HPV-positive oral and oropharyngeal squamous cell carcinomas. Biomed Res Int 2014;2014:303929.
- Qian X, Ma C, Nie X, et al. Biology and immunology of cancer stem(-like) cells in head and neck cancer. Crit Rev Oncol Hematol 2015;95:337-45. [Crossref] [PubMed]
- Krishnamurthy S, Nor JE. Head and neck cancer stem cells. J Dent Res 2012;91:334-40. [Crossref] [PubMed]
- Jewett A, Tseng HC, Arasteh A, et al. Natural killer cells preferentially target cancer stem cells; role of monocytes in protection against NK cell mediated lysis of cancer stem cells. Curr Drug Deliv 2012;9:5-16. [Crossref] [PubMed]
- Tang AL, Owen JH, Hauff SJ, et al. Head and neck cancer stem cells: the effect of HPV--an in vitro and mouse study. Otolaryngol Head Neck Surg 2013;149:252-60. [Crossref] [PubMed]
- Zhang M, Kumar B, Piao L, et al. Elevated intrinsic cancer stem cell population in human papillomavirus-associated head and neck squamous cell carcinoma. Cancer 2014;120:992-1001. [Crossref] [PubMed]
- Qian X, Wagner S, Ma C, et al. ALDH1-positive cancer stem-like cells are enriched in nodal metastases of oropharyngeal squamous cell carcinoma independent of HPV status. Oncol Rep 2013;29:1777-84. [PubMed]
- Chen YW, Chen KH, Huang PI, et al. Cucurbitacin I suppressed stem-like property and enhanced radiation-induced apoptosis in head and neck squamous carcinoma--derived CD44(+)ALDH1(+) cells. Mol Cancer Ther 2010;9:2879-92. [Crossref] [PubMed]
- Bourguignon LY, Wong G, Earle C, et al. Hyaluronan-CD44v3 interaction with Oct4-Sox2-Nanog promotes miR-302 expression leading to self-renewal, clonal formation, and cisplatin resistance in cancer stem cells from head and neck squamous cell carcinoma. J Biol Chem 2012;287:32800-24. [Crossref] [PubMed]
- Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol 2006;90:51-81. [Crossref] [PubMed]
- Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. J Clin Oncol 2015;33:3293-304. [Crossref] [PubMed]
- Leibowitz MS, Andrade Filho PA, Ferrone S, et al. Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol Immunother 2011;60:525-35. [Crossref] [PubMed]
- Wang T, Niu G, Kortylewski M, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 2004;10:48-54. [Crossref] [PubMed]
- Lalami Y, Awada A. Innovative perspectives of immunotherapy in head and neck cancer. From relevant scientific rationale to effective clinical practice. Cancer Treat Rev 2016;43:113-23. [Crossref] [PubMed]
- Gildener-Leapman N, Ferris RL, Bauman JE. Promising systemic immunotherapies in head and neck squamous cell carcinoma. Oral Oncol 2013;49:1089-96. [Crossref] [PubMed]
- Allen C, Duffy S, Teknos T, et al. Nuclear factor-kappaB-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clin Cancer Res 2007;13:3182-90. [Crossref] [PubMed]
- Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009;9:798-809. [Crossref] [PubMed]
- Sato T, Terai M, Tamura Y, et al. Interleukin 10 in the tumor microenvironment: a target for anticancer immunotherapy. Immunol Res 2011;51:170-82. [Crossref] [PubMed]
- Melero I, Rouzaut A, Motz GT, et al. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov 2014;4:522-6. [Crossref] [PubMed]
- Nagaraj S, Schrum AG, Cho HI, et al. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol 2010;184:3106-16. [Crossref] [PubMed]
- De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013;23:277-86. [Crossref] [PubMed]
- Balermpas P, Rodel F, Liberz R, et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br J Cancer 2014;111:1509-18. [Crossref] [PubMed]
- Fujii N, Shomori K, Shiomi T, et al. Cancer-associated fibroblasts and CD163-positive macrophages in oral squamous cell carcinoma: their clinicopathological and prognostic significance. J Oral Pathol Med 2012;41:444-51. [Crossref] [PubMed]
- Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res 2012;72:3125-30. [Crossref] [PubMed]
- Tsushima F, Tanaka K, Otsuki N, et al. Predominant expression of B7-H1 and its immunoregulatory roles in oral squamous cell carcinoma. Oral Oncol 2006;42:268-74. [Crossref] [PubMed]
- Cho YA, Yoon HJ, Lee JI, et al. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol 2011;47:1148-53. [Crossref] [PubMed]
- Skeate JG, Woodham AW, Einstein MH, et al. Current therapeutic vaccination and immunotherapy strategies for HPV-related diseases. Hum Vaccin Immunother 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Venuti A, Curzio G, Mariani L, et al. Immunotherapy of HPV-associated cancer: DNA/plant-derived vaccines and new orthotopic mouse models. Cancer Immunol Immunother 2015;64:1329-38. [Crossref] [PubMed]
- Schiller JT, Castellsague X, Villa LL, et al. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine 2008;26 Suppl 10:K53-61. [Crossref] [PubMed]
- Herrero R, Quint W, Hildesheim A, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One 2013;8:e68329. [Crossref] [PubMed]
- Gildener-Leapman N, Lee J, Ferris RL. Tailored immunotherapy for HPV positive head and neck squamous cell cancer. Oral Oncol 2014;50:780-4. [Crossref] [PubMed]
- Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol 2003;3:973-83. [Crossref] [PubMed]
- Voskens CJ, Sewell D, Hertzano R, et al. Induction of MAGE-A3 and HPV-16 immunity by Trojan vaccines in patients with head and neck carcinoma. Head Neck 2012;34:1734-46. [Crossref] [PubMed]
- Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine 2008;26:5315-20. [Crossref] [PubMed]
- June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest 2007;117:1466-76. [Crossref] [PubMed]
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78. [Crossref] [PubMed]
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116-27. [Crossref] [PubMed]
- Seidel UJ, Schlegel P, Lang P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front Immunol 2013;4:76. [Crossref] [PubMed]
- Vermorken JB, Stohlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol 2013;14:697-710. [Crossref] [PubMed]
- Saloura V, Cohen EE, Licitra L, et al. An open-label single-arm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 2014;73:1227-39. [Crossref] [PubMed]
- Basavaraj C, Sierra P, Shivu J, et al. Nimotuzumab with chemoradiation confers a survival advantage in treatment-naive head and neck tumors over expressing EGFR. Cancer Biol Ther 2010;10:673-81. [Crossref] [PubMed]
- Seiwert TY, Gupta S, Mehra R, et al. Antitumor activity and safety of pembrolizumab in patients (pts) with advanced squamous cell carcinoma of the head and neck (SCCHN): Preliminary results from KEYNOTE-012 expansion cohort. J Clin Oncol 2015;33:abstr LBA6008.
- Fury M, Ou SH, Balmanoukian A, et al. Clinical activity and safety of MEDI4736, an Anti-PD-L1 Antibody, In Head and Neck Cancer. ESMO Meeting 2014. Poster # 988PD. Abstract ID 5656.
- Campbell KS, Purdy AK. Structure/function of human killer cell immunoglobulin-like receptors: lessons from polymorphisms, evolution, crystal structures and mutations. Immunology 2011;132:315-25. [Crossref] [PubMed]
- Inoue K, Saegusa N, Omiya M, et al. Immunologically augmented skin flap as a novel dendritic cell vaccine against head and neck cancer in a rat model. Cancer Sci 2015;106:143-50. [Crossref] [PubMed]
- Schuler PJ, Harasymczuk M, Visus C, et al. Phase I dendritic cell p53 peptide vaccine for head and neck cancer. Clin Cancer Res 2014;20:2433-44. [Crossref] [PubMed]
- Whiteside TL, Ferris RL, Szczepanski M, et al. Dendritic cell-based autologous tumor vaccines for head and neck squamous cell carcinoma. Head Neck 2016;38 Suppl 1:E494-501. [Crossref] [PubMed]
- To WC, Wood BG, Krauss JC, et al. Systemic adoptive T-cell immunotherapy in recurrent and metastatic carcinoma of the head and neck: a phase 1 study. Arch Otolaryngol Head Neck Surg 2000;126:1225-31. [Crossref] [PubMed]
- Jiang P, Zhang Y, Wang H, et al. Adoptive cell transfer after chemotherapy enhances survival in patients with resectable HNSCC. Int Immunopharmacol 2015;28:208-14. [Crossref] [PubMed]
- Chia WK, Teo M, Wang WW, et al. Adoptive T-cell transfer and chemotherapy in the first-line treatment of metastatic and/or locally recurrent nasopharyngeal carcinoma. Mol Ther 2014;22:132-9. [Crossref] [PubMed]


