
Sacituzumab govitecan in triple-negative breast cancer
Antibody-drug conjugates (ADCs) represent a major and promising new sub-class of antibody-related therapeutics in oncology. They are composed of a monoclonal antibody (mAb) chemically linked (via the linker) to a cytotoxic drug, also known as the payload. Since 2013, the US FDA approved five ADCs in eight indications for the treatment of solid tumors (Table 1). Three of them are approved in breast cancer (BC): two trastuzumab-based ADCs in the HER2+ subtype (HER2+ BC) and one in the triple-negative subtype (TNBC), sacituzumab govitecan (SG). TNBCs represent 15% of cases and the most aggressive subtype of disease, for which the chemotherapy remained during a long time the only systemic treatment (1).
Table 1
| ADC | Target antigen | Linker type | Payload | FDA-approved indication [year of approval] |
|---|---|---|---|---|
| Trastuzumab emtansine (T-DM1) | HER2 | Non-cleavable | DM1 (microtubule inhibitor) | HER2+ advanced BC after trastuzumab and a taxane [2013] |
| HER2+ early BC if residual invasive disease after neoadjuvant treatment [2019] | ||||
| Trastuzumab deruxtecan (T-DXd) | HER2 | Cleavable | Deruxtecan (topoisomerase I inhibitor) | Advanced HER2+ BC after ≥2 lines in metastatic setting [2019] |
| HER2+ advanced gastric adenocarcinoma after trastuzumab-based regimen [2021] | ||||
| SG | TROP2 | Cleavable | SN-38 (topoisomerase I inhibitor) | Advanced TNBC after >2 lines [2020] |
| Metastatic urothelial cancer after platinum-based chemotherapy and immunotherapy [2021] | ||||
| Enfortumab vedotin | Nectin 4 | Cleavable | MMAE (microtubule inhibitor) | Advanced urothelial carcinoma after prior platinum-based chemotherapy and PD-1 or PD-L1 inhibitors [2020] |
| Tisotumab vedotin | TF | Cleavable | MMAE (microtubule inhibitor) | Recurrent or metastatic cervical cancer progressive on or after chemotherapy [2021] |
ADCs, antibody-drug conjugates; BC, breast cancer; SG, sacituzumab govitecan; TNBC; triple-negative breast cancer; TF, tissue factor.
SG combines a humanized mAb against human trophoblast cell-surface antigen 2 (TROP2), linked through a hydrolysable linker to SN-38, the active metabolite of irinotecan and a potent inhibitor of DNA topoisomerase 1. TROP2 is a transmembrane calcium signal transducer. Its function is not fully understood although it has previously been suggested to be involved in a variety of cell signaling pathways including proliferation, survival, self-renewal, and invasion (2-4). After administration, the anti-TROP2 ADC binds to TROP2 that is expressed on the tumor-cell surface. Upon ligation, the ADC is internalized via endocytosis, which allows for the targeted delivery of SN-38 into the tumor cells. Promising results from a small study led on April 2020 to the accelerated approval of SG in patients with advanced TNBC (5). More recently, Bardia et al. reported the results of the ASCENT phase III study assessing SG as compared to single-agent chemotherapy of the physician’s choice (eribulin, vinorelbine, capecitabine, or gemcitabine) in patients with relapsed or refractory metastatic TNBC. A total of 468 patients without brain metastases were enrolled and randomly assigned to receive SG (235 patients) or chemotherapy (233 patients). With a median follow-up of 17.7 months, the patients receiving SG had a median progression-free survival (PFS) of 5.6 vs. 1.7 months for those receiving a single-agent chemotherapy (HR =0.41; 95% CI: 0.32–0.52; P<0.001), associated with an improved median overall survival (OS) of 12.1 vs. 6.7 months (HR =0.48; 95% CI: 0.38−0.59; P<0.001) (6). The percentage of patients with an objective response (OR) was 35% with SG and 5% with the chemotherapy (OR =10.8; 95% CI: 5.6–21.0). The incidence of grade 3 or higher toxicities was higher in the SG arm than in the chemotherapy arm (neutropenia: 51% vs. 33%, leukopenia: 10% vs. 5%), diarrhea: 10% vs. <1%, anemia: 8% vs. 5%, and febrile neutropenia: 6% vs. 2%). These data confirmed the SG superiority over the chemotherapy in advanced stage TNBC and supported the FDA-accelerated approval’s upgrade to a regular approval. SG is thus currently approved for the patients with advanced TNBC previously treated with two or more lines of systemic therapy, including one in the metastatic setting. To date, no other ADC has been approved by the FDA in TNBC.
Identification of biomarkers predictive for the SG efficiency is warranted. A genomic (WES) and transcriptomic (RNA-seq) analysis of pre- and post-SG tumor samples from three patients with metastatic TNBC treated with SG was reported, the post-treatment samples corresponding to multisite progressions harvested at rapid autopsy (7): TROP2 expression was a pre-treatment determinant of response, whereas acquired mutations involving the direct targets of antibody (TROP2) and of drug payload (TOP1) were identified in one patient in the post-progression samples. The largest translational study of TNBC patients treated with SG is the biomarker analysis of ASCENT which focused on TROP2 expression and germline BRCA mutations. The trial was based on a patient population unselected for TROP2 expression (8,9). Immunohistochemistry (IHC) from mainly archival primary or metastatic tumor samples was centrally carried out; the results were available for 151 patients treated with SG and 139 treated with chemotherapy. Tumor cell membrane TROP2 expression was categorized based on the histochemical score (H-score), ranging from 0 to 300 and defining three expression categories (low, moderate, and high). The patients with high (~55% of cases) and moderate (~25%) TROP2 expression showed higher ORs rate and longer PFS than patients with low expression (20%). However, these latter still had improved PFS with SG compared to chemotherapy, but the sample size was too small to make a definitive conclusion. These results suggest that assessment of TROP2 expression may not be needed to predict which patients are unlikely to derive benefit from SG vs. chemotherapy. However, the small number of patients in the low expression group, the lack of validated IHC assay, and the analysis mainly limited to archival samples although TROP2 may be a dynamic biomarker call for additional studies to address the predictive value of its expression. Clearly, the ongoing development and comparison of different assays (10,11) are warranted to optimally quantify TROP2 expression, and perhaps to help refining its predictive value for SG benefit. Such value is also challenged by the pathological context, with for example conflictual results observed in the metastatic small cell lung cancer (mSCLC) with no clear relationship between TROP2 expression and PFS or OS (12). Interestingly, a TROP2 expression-independent clinical activity of another anti-TROP2 ADC, datopotamab-deruxtecan, was also reported in the non-small cell lung cancer (NSCLC) cohort from the TROPION-PanTumor01 phase I study (13). Furthermore, other mechanisms of resistance, suggested with other ADCs (intratumor heterogeneity, elevated drug transporters such as MDR1 and MRP1, altered antibody trafficking, ADC processing, intracellular drug release, alteration of the payload target…) (14), certainly exist for SG. The search for biomarkers predictive for response/resistance is crucial and calls for the development of preclinical models of resistance and the storage of pre- and post-treatment clinical samples.
In their paper, Bardia et al. (6) mentioned that “TROP2 is highly expressed in multiple tumor types, including the breast cancer (>90%)”. However, and in a very surprising way, the three studies they were referring to do not support this statement in BC (15-17). Indeed, in the first reference, Ripani et al. (15) focused on the cytosolic Ca2+ measurement using two cell lines including one BC cell line. In the second one, a review article from Zaman et al. (16), two main studies are mentioned: one published in Science on 1997 (18) using the serial analysis of gene expression method in human colorectal epithelium, colorectal cancers, and pancreatic cancers; the other study published in Scientific Reports on 2016 was a meta-analysis of the prognostic impact studies based on the TROP2 expression by immunochemistry or RT-qPCR in cholangiocarcinoma, gastric, nasopharyngeal, gallbladder, cervical, non-small cell lung, laryngeal squamous cell, endometrial, ovarian, intestinal-type, squamous cell, and colorectal cancers, but not in BC samples (19). The third reference (17) mentioned by Bardia et al. focused on the RS7 antibody targeting TROP2 and immunoprecipitation analyses, showing in vitro serine protein kinase C phosphorylation of the protein, without any data on clinical BC samples.
Given this lack of compelling data justifying the Bardia et al.’s statements, we attempted to examine the TROP2 expression in clinical BC samples and other cancer types in comparison with the corresponding normal tissues. First, we used the cancer cell lines data from the Dependency Map (DepMap) portal (https://depmap.org/portal) to compare the mass spectrometry-based protein expression to RNA-seq-based mRNA expression of TROP2 (20,21). The TROP2 mRNA expression was strongly correlated with the protein expression in the series of 369 cancer cell lines (r=0.84, P=3.55E-101), including 30 BC cell lines (r=0.77, P=7.16E-07). Then, we analyzed The Cancer Genome Atlas Program (TCGA) gene expression data in 33 cancer types and normal corresponding tissues using the GEPIA2 tool (22). When compared to the normal tissues, TROP2 was overexpressed in the bladder urothelial carcinoma, cervical squamous cell carcinoma and endocervical adenocarcinoma, colon adenocarcinoma, lung adenocarcinoma, lung squamous cell carcinoma, ovarian serous cystadenocarcinoma, pancreatic adenocarcinoma, prostate adenocarcinoma, rectum adenocarcinoma, stomach adenocarcinoma, thymoma, thyroid carcinoma, thymoma, and uterine carcinosarcoma. Underexpression in the tumors compared to normal was observed in esophageal carcinoma, kidney chromophobe, kidney renal clear cell carcinoma, and skin cutaneous melanoma. No significant difference in expression between the tumors and normal tissues was observed in adrenocortical carcinoma, invasive breast carcinoma, cholangiocarcinoma, diffuse large B-cell lymphoma, glioblastoma multiforme, head and neck squamous cell carcinoma, kidney renal papillary cell carcinoma, acute myeloid leukemia, brain lower grade glioma, liver hepatocellular carcinoma, mesothelioma, pheochromocytoma and paraganglioma, sarcoma, testicular germ cell tumors, and uveal melanoma (Figure 1).
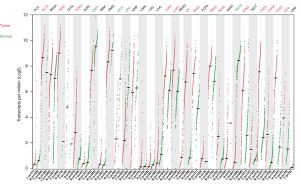
These data, including 1,085 BC clinical samples and 291 paired normal tissue samples, do not support TROP2 overexpression, at least at the mRNA level and in BC compared to unpaired normal mammary tissues. In fact, very few data are available regarding the mRNA or protein TROP2 expression in paired normal and tumor BC tissue samples. Two studies showed higher expression in the BC samples than in the tumor-adjacent non-malignant tissues at the mRNA level in 15 pairs (23) and 20 pairs (24), and at the protein level in 59 pairs (23) and 70 pairs (24). Another study showed a similar result at the mRNA level in 50 pairs (25).
Thus, the data in TCGA tumors challenge the notion of TROP2 as an ideal antigen target highly expressed at the surface of cancer cells with low expression on healthy tissues to limit the on-target off-tumor toxicity (26). Nevertheless, the lack of relative abundance of antigen expression compared with normal tissue does not preclude ADC activity. In some cases, low antigen expression may still function (i.e., DS-8201 activity in low HER2 tumors) depending on antibody-antigen affinity, cell membrane permeability, or linker stability (27). However, some ADCs act also by mediating the so-called bystander killing effect, by which the non-target-expressing cells neighboring the targeted cells are killed either by extraneous drug expelled from cells targeted by the ADC, or by the extracellular release of the payload by early cleavage of the linker (28). The linker attaching the mAb to SN-38 in SG contains a protease site that was anticipated to be cleaved by lysosomal enzymes to release SN-38 within the cell. However, the ADC releases SN-38 with a half-life measured in serum of 17.5 hours with close clearance time for SG (~14 hours) (29), suggesting that SG might also act as an SN-38 prodrug in addition to a conventional ADC. Such hypothesis might explain the SG efficiency independently from the TROP2 expression and the SG systemic toxicity. This bystander effect might represent a promising avenue for the future development of ADCs by focusing on the development of combinations to exploit the tumor microenvironment such conjugating ADCs with immune-stimulant molecules as a second payload and/or to synergize with immune checkpoint inhibition.
In conclusion, even if its mechanism of action remains debated, SG represents a major advance in the treatment of advanced TNBC. Because TROP2 is widely expressed in TNBC and thanks to the strong bystander effect of SG, this latter should lead to better efficacy in the whole population of TNBC patients than other drugs recently approved, efficient but only in patients’ subgroups such as the 5–10% of patients with germline BRCA mutations for the PARP inhibitors (olaparib, talazoparib) and the 40% of patients with a combined positive score (CPS) ≥10% for the pembrolizumab immune checkpoint inhibitor. Clinical trials assessing SG, alone or in combination, are ongoing in early TNBC in both the neo-adjuvant setting (NeoSTAR trial: NCT04230109) and the post-neo-adjuvant setting (SASCIA trial: NCT04595565). Because TROP2 is also expressed in the HR+/HER2− BC, the TROPiCS-02 phase III trial recently compared SG with the physician’s choice of chemotherapy in patients with metastatic HR+/HER2− BC (30) with positive results for its primary endpoint (PFS). Other ADCs are in development in advanced TNBC such as datopotamab-deruxtecan directed against TROP2 and other ADCs directed against nectin4 (31) and LIV1 (32).
Acknowledgments
Funding: Our work was supported by Institut Paoli-Calmettes, la Ligue Nationale Contre le Cancer (Label Ligue EL2022 to FB), and Le Prix Ruban Rose 2020 (to FB). None of them had any role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Translational Medicine. The article has undergone external peer review.
Peer Review File: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-813/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-813/coif). FB is supported by Institut Paoli-Calmettes, la Ligue Nationale Contre le Cancer (Label Ligue EL2022) and Le Prix Ruban Rose 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bertucci F, Finetti P, Birnbaum D. Basal breast cancer: a complex and deadly molecular subtype. Curr Mol Med 2012;12:96-110. [Crossref] [PubMed]
- Cubas R, Zhang S, Li M, et al. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol Cancer 2010;9:253. [Crossref] [PubMed]
- Guerra E, Trerotola M. A bicistronic CYCLIN D1-TROP2 mRNA chimera demonstrates a novel oncogenic mechanism in human cancer. Cancer Res 2008;68:8113-21. [Crossref] [PubMed]
- Wang J, Day R, Dong Y, et al. Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol Cancer Ther 2008;7:280-5. [Crossref] [PubMed]
- Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med 2019;380:741-51. [Crossref] [PubMed]
- Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med 2021;384:1529-41. [Crossref] [PubMed]
- Coates JT, Sun S, Leshchiner I, et al. Parallel Genomic Alterations of Antigen and Payload Targets Mediate Polyclonal Acquired Clinical Resistance to Sacituzumab Govitecan in Triple-Negative Breast Cancer. Cancer Discov 2021;11:2436-45. [Crossref] [PubMed]
- Bardia A, Tolaney SM, Punie K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol 2021;32:1148-56. [Crossref] [PubMed]
- Hurvitz SA, Tolaney SM, Punie K, et al. Abstract GS3-06: Biomarker evaluation in the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Cancer Res 2021;81:GS3-06.
- Sayama Y, Kaneko MK, Kato Y. Development and characterization of TrMab 6, a novel anti TROP2 monoclonal antibody for antigen detection in breast cancer. Mol Med Rep 2021;23:92. [Crossref] [PubMed]
- Sayama Y, Kaneko MK, Takei J, et al. Establishment of a novel anti-TROP2 monoclonal antibody TrMab-29 for immunohistochemical analysis. Biochem Biophys Rep 2021;25:100902. [Crossref] [PubMed]
- Gray JE, Heist RS, Starodub AN, et al. Therapy of Small Cell Lung Cancer (SCLC) with a Topoisomerase-I-inhibiting Antibody-Drug Conjugate (ADC) Targeting Trop-2, Sacituzumab Govitecan. Clin Cancer Res 2017;23:5711-9. [Crossref] [PubMed]
- Meric-Bernstam F, Spira AI, Lisberg AE, et al. TROPION-PanTumor01: Dose analysis of the TROP2-directed antibody-drug conjugate (ADC) datopotamab deruxtecan (Dato-DXd, DS-1062) for the treatment (Tx) of advanced or metastatic non-small cell lung cancer (NSCLC). J Clin Oncol 2021;39:9058. [Crossref]
- Loganzo F, Sung M, Gerber HP. Mechanisms of Resistance to Antibody-Drug Conjugates. Mol Cancer Ther 2016;15:2825-34. [Crossref] [PubMed]
- Ripani E, Sacchetti A, Corda D, et al. Human Trop-2 is a tumor-associated calcium signal transducer. Int J Cancer 1998;76:671-6. [Crossref] [PubMed]
- Zaman S, Jadid H, Denson AC, et al. Targeting Trop-2 in solid tumors: future prospects. Onco Targets Ther 2019;12:1781-90. [Crossref] [PubMed]
- Basu A, Goldenberg DM, Stein R. The epithelial/carcinoma antigen EGP-1, recognized by monoclonal antibody RS7-3G11, is phosphorylated on serine 303. Int J Cancer 1995;62:472-9. [Crossref] [PubMed]
- Zhang L, Zhou W, Velculescu VE, et al. Gene expression profiles in normal and cancer cells. Science 1997;276:1268-72. [Crossref] [PubMed]
- Zeng P, Chen MB, Zhou LN, et al. Impact of TROP2 expression on prognosis in solid tumors: A Systematic Review and Meta-analysis. Sci Rep 2016;6:33658. [Crossref] [PubMed]
- DepMap. Broad DepMap 21Q4 Public, 2021.
- Nusinow DP, Szpyt J, Ghandi M, et al. Quantitative Proteomics of the Cancer Cell Line Encyclopedia. Cell 2020;180:387-402.e16. [Crossref] [PubMed]
- Tang Z, Kang B, Li C, et al. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res 2019;47:W556-60. [Crossref] [PubMed]
- Lin H, Huang JF, Qiu JR, et al. Significantly upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of invasive ductal breast cancer. Exp Mol Pathol 2013;94:73-8. [Crossref] [PubMed]
- Zhao W, Kuai X, Zhou X, et al. Trop2 is a potential biomarker for the promotion of EMT in human breast cancer. Oncol Rep 2018;40:759-66. [Crossref] [PubMed]
- Trerotola M, Cantanelli P, Guerra E, et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene 2013;32:222-33. [Crossref] [PubMed]
- Chau CH, Steeg PS, Figg WD. Antibody-drug conjugates for cancer. Lancet 2019;394:793-804. [Crossref] [PubMed]
- Modi S, Park H, Murthy RK, et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol 2020;38:1887-96. [Crossref] [PubMed]
- Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer 2017;117:1736-42. [Crossref] [PubMed]
- Sharkey RM, McBride WJ, Cardillo TM, et al. Enhanced Delivery of SN-38 to Human Tumor Xenografts with an Anti-Trop-2-SN-38 Antibody Conjugate (Sacituzumab Govitecan). Clin Cancer Res 2015;21:5131-8. [Crossref] [PubMed]
- Rugo HS, Bardia A, Tolaney SM, et al. TROPiCS-02: A Phase III study investigating sacituzumab govitecan in the treatment of HR+/HER2- metastatic breast cancer. Future Oncol 2020;16:705-15. [Crossref] [PubMed]
- M-Rabet M. Nectin-4: a new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann Oncol 2017;28:769-76. [Crossref] [PubMed]
- Sussman D, Smith LM, Anderson ME, et al. SGN-LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther 2014;13:2991-3000. [Crossref] [PubMed]


