
The relationship between transthoracic echocardiography and mortality in adult patients with multiple organ dysfunction syndrome: analysis of the MIMIC-III database
Introduction
Many patients in the intensive care unit (ICU) experience multiple organ dysfunction syndrome (MODS), for which timely assessment and real-time adjustment of the treatment plan are critically important (1-3). During their stay in the ICU, patients with MODS receive a wide array of blood tests, examinations, and monitoring that provide doctors with the required information for treatment (4,5). As a simple and noninvasive examination method, ultrasound is widely used in clinical practice (6,7), with cardiac ultrasound being particularly valuable for examining patients with heart disease or severe disease (8-10). Cardiac ultrasound is commonly used in clinical practice through two main approaches: transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) (10-12). The ease in which TTE can be operated facilitates patient cooperation and a broader range of clinical applications (13). However, the measurement’s from TTE are easily affected by the features of a given patient’s chest wall, lungs, and other anatomical sites. In contrast, with TEE examination, the probe is placed near to the left atrium, which provides excellent accuracy (11); in particular, the accuracy in detecting intracardiac thrombosis is significantly higher in TEE than in TTE (11). However, as the operation of TEE is more complicated and requires a high degree of patient cooperation, it is less commonly used (11). Nonetheless, in some patients, even when a variety of examinations is performed, the patients’ condition cannot be accurately assessed, or the treatment cannot be adjusted according to the examination results, rendering some examinations and tests clinically irrelevant (14). At the same time, overuse of examination or intervention is prevalent world widely, which cause heavy burden on patients and government. In this study, we aimed to investigate whether the application of TTE in the ICU can improve the short-term prognosis of MODS patients. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-717/rc).
Methods
Data source and study population
This study used a retrospective design, in which data from the Medical Information Mart for Intensive Care III (MIMIC-III v1.4) (15) database were extracted and analyzed. The MIMIC-III database holds clinical information of more than 50,000 patients who were hospitalized in the ICU of Beth Israel Dikang Medical Center in the United States from June 2001 to October 2012. The data include information on vital signs, drugs used, laboratory test results, imaging examination reports, nursing records, fluid access and type, diagnosis code, length of stay, clinical outcome, etc. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
The patients in this study were those with MODS who were hospitalized in the ICU. The inclusion criteria for patients were the following: (I) age ≥18 years, (II) a first-time admission to the ICU (for those who had been admitted to the ICU multiple times, only the information of first stay in ICU was recorded), (III) a first-time diagnosis of MODS, and (IV) a stay time in the ICU of ≥24 hours. The exclusion criteria were the following: (I) the missing of key information, such as lack of scores for severe illness; and (II) patients who were discharged automatically.
Data extraction
Structured Query Language (SQL) was used to extract the following data from the MIMIC-III database according to previous studies (16,17): age, sex, body weight, white blood cell, hemoglobin, platelet, blood urine nitrogen, creatine, blood glucose, electrolytes, blood HCO3−, sequential organ failure assessment (SOFA), and ICU hospitalization duration and death. Other data extracted included information concerning comorbidities (hypertension, coronary heart disease, chronic obstructive pulmonary disease (COPD); chronic kidney disease; the use of mechanical ventilation, renal replacement therapy, or vasoactive drugs during ICU hospitalization; and complications including ventilator-associated pneumonitis, urinary tract infection, diabetic ketone acidosis, and acute myocardial infarction. We only extracted laboratory test results generated within the first 24 hours after ICU admission and the maximum value (max) and minimum value (min) during ICU stay period.
Outcomes
The primary outcome in the present study was the all-cause death within 28 days after ICU admission. The secondary outcomes included the number of ventilation-free days within 28 days after ICU admission, the number of days of vasopressors, the maximum dose of norepinephrine, the volume of fluid injected in the first, second and third day after ICU admission, the level of serum lactate, and the rate of creatinine decrease. For patients undergoing TTE examination, the calculation of lactate and creatinine decrease was performed as follows: the last test result before TTE examination—the first test result 48 hours after TTE examination; for patients who did not receive TTE, this decrease was calculated as follows: the first test result after ICU admission—the result of the first test 48 hours after this test.
Statistical analysis
SPSS software (version 23.0, IBM Corp., Armonk, NY, USA) was used to analyze extracted data. Continuous variables are expressed as mean and SD, and comparison between the two groups were conducted using independent-sample t-tests. Categorical variables are expressed as percentages (%), and comparison between the two groups were conducted using the χ2 test. Multivariate regression analysis was conducted to infer the risk factors associated with the primary outcome. Double robust analysis was conducted to investigate the correlation between the presence of a TTE check and patient outcomes. A gradient-boosted model (GBM) was constructed to calculate the propensity score (PS) of patients received TTE examination to reduce the imbalance of variables between these two groups. Weighted with PS, an inverse probabilities weighting (IPW) model was constructed to generate a weighted queue. A two-sided P value <0.05 indicated statistical difference.
Results
General information
We finally extracted 13,844 patients with MODS from the MIMIC-III database (Figure 1) and divided them into a TTE group and a non-TTE group. There were 5,022 cases (36.28%) in the TTE group, 2,416 (48.10%) of whom were female; and 8,822 cases (63.72%) in the non-TTE group, 4,129 (46.80%) of whom were female. Compared with non-TTE group, the simplified acute physiological score (SAPS) score (20.07±5.28 vs. 17.97±5.33; t=22.364; P<0.001) and SOFA score (5.98±3.41 vs. 4.60±2.78; t=25.818; P<0.001) of patients in the TTE group were higher. The portion of patients in the TTE group receiving mechanical ventilation (59.00% vs. 40.00%) and vasopressors (32.40% vs. 16.10%) was significantly higher than that in the non-TTE group. These data suggested that patients in TTE group were more severely ill than those in non-TTE group (Table 1).
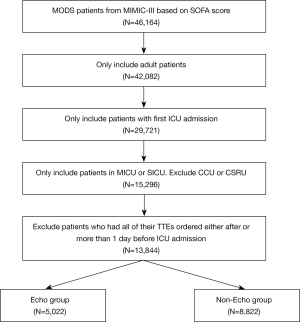
Table 1
| Covariate | Nonecho | Echo | SMD | Nonecho (PS matching) | Echo (PS matching) | SMD (PS matching) | Missing data (%) |
|---|---|---|---|---|---|---|---|
| N | 8,822 | 5,022 | 3,395 | 3,395 | |||
| Age (years) | 64.04 (18.05) | 66.24 (17.00) | 0.125 | 66.82 (17.23) | 65.27 (17.57) | 0.089 | 0 |
| Gender (female, %) | 46.80 | 48.10 | 0.026 | 45.70 | 48.10 | 0.050 | 0 |
| Service unit (MICU, %) | 66.30 | 75.10 | 0.194 | 72.50 | 72.40 | 0.004 | 0 |
| Weight (kg) | 79.00 (26.16) | 82.39 (25.77) | 0.131 | 81.28 (29.47) | 80.61 (23.57) | 0.025 | 10.20 |
| SAPS score | 17.97 (5.33) | 20.07 (5.28) | 0.397 | 19.81 (5.29) | 19.16 (5.13) | 0.125 | 0 |
| SOFA score | 4.60 (2.78) | 5.98 (3.41) | 0.443 | 5.57 (3.30) | 5.32 (3.13) | 0.075 | 0 |
| Elixhauser score | 8.13 (7.82) | 11.53 (8.27) | 0.422 | 10.48 (7.87) | 10.22 (8.12) | 0.032 | 0.30 |
| Mechanical ventilation use (first 24 hours, %) | 40.00 | 59.00 | 0.386 | 52.60 | 50.60 | 0.040 | 0 |
| Vasopressor use (first 24 hours, %) | 16.10 | 32.40 | 0.387 | 26.70 | 25.30 | 0.032 | 0 |
| Sedative use (first 24 hours, %) | 32.10 | 42.70 | 0.220 | 39.50 | 35.70 | 0.077 | 0 |
| CHF (%) | 14.30 | 39.30 | 0.590 | 29.90 | 26.20 | 0.081 | 0 |
| AFIB (%) | 16.90 | 31.50 | 0.346 | 27.20 | 25.50 | 0.038 | 0 |
| Renal (%) | 12.10 | 17.20 | 0.145 | 17.80 | 15.10 | 0.073 | 0 |
| Liver (%) | 10.50 | 9.80 | 0.022 | 11.20 | 9.90 | 0.041 | 0 |
| COPD (%) | 11.50 | 16.20 | 0.137 | 16.00 | 13.80 | 0.062 | 0 |
| CAD (%) | 12.20 | 17.20 | 0.141 | 16.60 | 16.30 | 0.006 | 0 |
| Stroke (%) | 11.10 | 13.20 | 0.066 | 12.50 | 14.00 | 0.044 | 0 |
| Malignancy (%) | 26.50 | 21.60 | 0.114 | 23.60 | 22.80 | 0.020 | 0 |
| Day of ICU admission (%) | 0.149 | 0.058 | 0 | ||||
| Sunday | 12.20 | 13.60 | 13.30 | 13.60 | |||
| Monday | 13.30 | 14.60 | 13.40 | 14.30 | |||
| Tuesday | 14.20 | 16.20 | 15.30 | 15.80 | |||
| Wednesday | 14.90 | 16.20 | 15.20 | 15.80 | |||
| Thursday | 15.20 | 15.40 | 15.10 | 14.90 | |||
| Friday | 17.20 | 12.90 | 14.60 | 14.10 | |||
| Saturday | 13.10 | 11.00 | 13.10 | 11.50 | |||
| Hour of ICU admission | 0.117 | 0.086 | 0 | ||||
| MAP (mmHg) | 83.19 (18.75) | 81.09 (19.54) | 0.110 | 81.75 (19.74) | 81.83 (19.31) | 0.004 | 0.40 |
| Heart rate (beats per minute) | 89.34 (19.54) | 93.10 (21.77) | 0.182 | 91.93 (20.81) | 91.99 (20.72) | 0.003 | 0.40 |
| Temperature (℃) | 36.72 (1.32) | 36.75 (1.64) | 0.024 | 36.73 (1.47) | 36.74 (1.47) | 0.012 | 0.80 |
| CVP (cmH2O) | 11.01 (13.20) | 13.75 (21.32) | 0.155 | 11.41 (11.36) | 12.52 (18.93) | 0.071 | 75.50 |
| WBC (×109) | 12.06 (14.42) | 13.13 (13.68) | 0.077 | 12.92 (14.24) | 12.95 (14.59) | 0.002 | 4.50 |
| Hb (g/L) | 10.87 (2.01) | 10.73 (2.06) | 0.067 | 10.72 (2.04) | 10.80 (2.06) | 0.039 | 4.40 |
| PLT (×109) | 214.85 (118.89) | 210.25 (123.43) | 0.038 | 213.76 (122.02) | 212.50 (126.10) | 0.010 | 4.40 |
| Sodium (mmol/L) | 138.98 (5.65) | 138.58 (5.62) | 0.069 | 138.92 (5.70) | 138.62 (5.61) | 0.053 | 3.10 |
| Potassium (mmol/L) | 4.10 (0.75) | 4.17 (0.82) | 0.092 | 4.17 (0.80) | 4.13 (0.83) | 0.048 | 2.90 |
| Bicarbonate (mmHg) | 23.14 (4.82) | 22.76 (5.55) | 0.072 | 22.88 (5.51) | 22.77 (5.19) | 0.019 | 3.40 |
| Chloride (mmol/L) | 105.69 (6.75) | 104.92 (6.90) | 0.112 | 105.25 (7.05) | 105.09 (6.84) | 0.023 | 3.20 |
| BUN (mmol/L) | 27.09 (23.14) | 34.05 (26.00) | 0.283 | 32.99 (26.10) | 31.45 (24.97) | 0.060 | 3.40 |
| Lactate (mmol/L) | 2.49 (2.23) | 2.51 (2.31) | 0.007 | 2.62 (2.36) | 2.59 (2.48) | 0.012 | 52.70 |
| Creatinine (mmol/L) | 1.46 (1.74) | 1.82 (1.88) | 0.198 | 1.76 (2.02) | 1.73 (1.92) | 0.013 | 3.30 |
| PH | 7.36 (0.10) | 7.35 (0.11) | 0.129 | 7.35 (0.11) | 7.35 (0.11) | 0.053 | 42.50 |
| PO2 (mmHg) | 153.13 (103.69) | 135.10 (96.84) | 0.180 | 143.74 (105.67) | 143.29 (100.91) | 0.004 | 45.40 |
| PCO2 (mmHg) | 41.60 (12.73) | 42.76 (14.59) | 0.084 | 42.58 (14.48) | 41.58 (13.73) | 0.071 | 45.40 |
| BNP (tested, %) | 0.70 | 3.90 | 0.216 | 1.60 | 1.40 | 0.017 | 0 |
| Troponin (tested, %) | 14.90 | 40.90 | 0.604 | 32.00 | 29.50 | 0.054 | 0 |
| Creatinine kinase (tested, %) | 32.50 | 61.60 | 0.610 | 55.20 | 51.50 | 0.073 | 0 |
Continuous data are expressed as mean and SD. ICU, intensive care unit; SMD, standardized mean difference; PS, propensity score; MICU, medical ICU; SAPS, simplified acute physiological score; SOFA, sequential organ failure score; CHF, chronic heart failure; AFIB, atrial fibrillation; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; MAP, mean arterial pressure; CVP, central venous pressure; WBC, white blood cell; Hb, hemoglobin; PLT, platelet; PO2, partial pressure of oxygen; PCO2, partial pressure of carbon dioxide; BUN, blood urea nitrogen; BNP, brain natriuretic peptide.
Double robust analysis
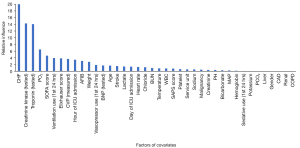
The results of PS analysis on covariates are shown in Figure 2, from which we can see the contribution of different covariates to the PS score. The covariates that contributed significantly to PS in this study included chronic heart failure (CHF), creatine kinase (CK), troponin, partial pressure of oxygen (PO2), and SOFA score, among others. Accordingly, the difference between the TTE group and the non-TTE group was standardized by IPW (15), and the results are shown in Table 1. Analyzing the matched cohort, we found that there are still some differences in many parameters between the two groups of patients, but the comparison results of some data were changed. For example, the portion of CHF patients in the TTE group was higher in the original cohort, while the portion of CHF patients in the non-TTE group was higher in the new cohort. Similar variables included SOFA score, mechanical ventilation, the use of drugs that raise blood pressure, the use of sedative drugs, the rate of atrial fibrillation (AFIB), the rate of myocardial injury biomarkers detection, etc.
Endpoint analysis
Double robust analysis showed that the risk of death within 28 hours after ICU admission in patients received TTE (21.0%) was lower than that in the non-TTE group (26.5%). The adjusted odds ratio (OR) value was 0.73 [95% confidence interval (CI): 0.65–0.82; P<0.001]. Meantime, three other models had similar results, further support that TTE check could reducing the risk of 28-day mortality (Table 2). Analysis of the secondary outcomes revealed that the time that patients received TTE group were ventilation-free (20.87±23.55 vs. 19.55±12.15; P=0.003) or vasopressors-free (21.87±13.46 vs. 20.36±12.28; P<0.001) was significantly longer than that in the non-TTE group; meanwhile the portion of patients used and the highest dose of norepinephrine used in the TTE group were higher than those in the non-TTE group, but the decrease in SOFA on the second and third days and the 48-hour decrease in serum lactic acid and serum creatinine were lower than those in the TTE group (Table 3).
Table 2
| Methods | OR | CI (2.5%) | CI (97.5%) | P value |
|---|---|---|---|---|
| Doubly robust with unbalanced covariates | 0.72 | 0.58 | 0.90 | <0.001 |
| Doubly robust with all covariates | 0.66 | 0.56 | 0.78 | <0.001 |
| PS (IPW) | 0.86 | 0.81 | 0.91 | <0.001 |
| PS matching | 0.73 | 0.65 | 0.82 | <0.001 |
| Multivariate | 0.68 | 0.58 | 0.8 | <0.001 |
OR, odds ratio; CI, confidence interval; PS, propensity score; IPW, inverse probabilities weighting.
Table 3
| Covariate | Nonecho | Echo | SMD | P value (multivariate) | Nonecho (weighted cohort) | Echo (weighted cohort) | SMD (weighted cohort) | P value (IPW) |
P value (doubly robust all covariates) | P value (doubly robust unbalanced covariates) | Nonecho (PS matching) | Echo (PS matching) | SMD (PS matching) | P value (PS matching) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 8,822 | 5,022 | 13,369.42 | 12,469.67 | 3,395 | 3,395 | ||||||||
| Ventilation-free days in 28 days | 21.99 (11.00) | 19.54 (21.20) | 0.145 | 0.051 | 20.90 (11.66) | 20.91 (19.51) | <0.001 | 0.988 | 0.091 | 0.355 | 19.55 (12.15) | 20.87 (23.55) | 0.071 | 0.003 |
| Vasopressor-free days in 28 days | 22.55 (11.06) | 21.01 (13.65) | 0.124 | <0.001 | 21.55 (11.76) | 22.09 (12.84) | 0.044 | 0.026 | <0.001 | 0.023 | 20.36 (12.28) | 21.87 (13.46) | 0.118 | <0.001 |
| Dobutamine use (%) | 0.30 | 3.60 | 0.236 | <0.001 | 0.60 | 2.30 | 0.144 | <0.001 | <0.001 | <0.001 | 0.80 | 2.20 | 0.113 | <0.001 |
| IV fluid day 1 (mL) | 1,273.85 (2,829.16) | 1,977.87 (3,638.30) | 0.216 | 0.212 | 1,402.99 (2,962.24) | 1,686.90 (3,343.43) | 0.090 | <0.001 | 0.201 | 0.149 | 1,622.42 (3,251.36) | 1,748.19 (3,461.69) | 0.037 | 0.180 |
| IV fluid day 2 (mL) | 438.38 (2,120.60) | 953.69 (2,724.98) | 0.211 | <0.001 | 516.57 (2,207.40) | 827.67 (2,583.04) | 0.129 | <0.001 | 0.004 | 0.005 | 638.99 (2,290.85) | 839.32 (2,660.75) | 0.081 | 0.032 |
| IV fluid day 3 (mL) | 49.74 (2,018.70) | 453.16 (2,483.46) | 0.178 | <0.001 | 103.94 (2,067.82) | 414.27 (2,384.94) | 0.139 | <0.001 | <0.001 | 0.001 | 183.17 (2,099.50) | 409.03 (2,416.58) | 0.100 | 0.052 |
| SOFA reduction day 2 | 0.93 (3.63) | 0.62 (3.01) | 0.092 | 0.008 | 0.89 (3.88) | 0.61 (2.83) | 0.081 | <0.001 | 0.089 | 0.121 | 0.87 (4.14) | 0.57 (3.00) | 0.084 | <0.001 |
| SOFA reduction day 3 | 1.15 (3.39) | 0.82 (3.12) | 0.100 | <0.001 | 1.19 (3.58) | 0.74 (2.94) | 0.134 | <0.001 | <0.001 | <0.001 | 1.25 (3.74) | 0.70 (3.06) | 0.159 | <0.001 |
| Norepinephrine (maximum dosage mg/min) | 0.39 (1.75) | 1.32 (4.80) | 0.256 | 0.003 | 0.57 (2.15) | 0.95 (3.81) | 0.125 | <0.001 | <0.001 | 0.029 | 0.73 (2.40) | 0.96 (2.98) | 0.085 | <0.001 |
| Serum lactate reduction (48 hours) | 0.70 (2.41) | 0.28 (1.89) | 0.192 | <0.001 | 0.69 (2.53) | 0.20 (1.84) | 0.221 | <0.001 | <0.001 | 0.001 | 0.68 (2.55) | 0.22 (1.94) | 0.201 | 0.318 |
| Serum creatinine reduction (48 hours) | 0.16 (0.93) | 0.13 (0.86) | 0.037 | 0.032 | 0.16 (0.95) | 0.12 (0.79) | 0.050 | 0.020 | 0.085 | 0.124 | 0.19 (1.17) | 0.12 (0.81) | 0.070 | 0.010 |
| Serum lactate reduction (24 hours) | 0.64 (2.13) | 0.17 (1.59) | 0.246 | <0.001 | 0.60 (2.22) | 0.11 (1.54) | 0.252 | <0.001 | <0.001 | <0.001 | 0.57 (2.28) | 0.13 (1.57) | 0.225 | 0.283 |
| Serum creatinine reduction (24 hours) | 0.11 (0.76) | 0.05 (2.23) | 0.038 | 0.077 | 0.11 (0.77) | 0.05 (1.98) | 0.038 | 0.050 | 0.152 | 0.171 | 0.13 (0.99) | 0.08 (0.66) | 0.056 | 0.028 |
Continuous data are expressed as mean and SD. SMD, standardized mean difference; IPW, inverse probabilities weighting; PS, propensity score; IV, intravenous; SOFA, sequential organ failure assessment.
Discussion
Through the mining and analysis of the MIMIC-III database, we found the use of TTE for patients with MODS to be associated with lower ICU mortality. In this study, as a whole, the condition of patients undergoing TTE examination was significantly worse than that of patients who did not undergo TTE examination; furthermore, the TTE group had a higher SAPS, SOFA, and Elixhauser score, as well as a higher portion of patients receiving mechanical ventilation, vasoactive drugs on the first day after ICU admission, and sedative drugs. The portion of patients in the TTE group with heart failure, AFIB, decreased renal function, COPD, coronary heart disease, and stroke was higher than that of the non-TTE group. Moreover, there was a higher portion of patients in the TTE group who were administered dopamine (Table 3), and patients in the TTE group received more intravenous (IV) fluids on the first, second, and third days after ICU admission than did those in the non-TTE group (although the difference between the first day and the third day was not statistically significant). Additionally, the maximum dose of norepinephrine in the TTE group was significantly higher than that in the non-TTE group. The results of this study were consistent with those of other studies (16,18).
MODS mainly affects the heart, lungs, liver, and kidneys. A decline in the function of these organs can lead to secondary changes in the functions of other organs. After the primary factors are corrected in a short period of time, most of the organ functions can be restored (4,19). Once the secondary decline of organ function precipitates substantial damage, the restoration of organ function becomes exceedingly challenging (20). Timely evaluation of the structural and functional changes of the primary organs and secondary organs is critical for assessing the patient’s condition and prognosis. Furthermore, a differential diagnosis can be completed to determine whether the damage of the target organ is primary or secondary (21). Heart failure is the most common cause of death in most patients with MODS. Timely assessment of the structure and function of the heart is thus essential for identifying those patients with decreased cardiac function and for guiding clinical decision-making (22). Cardiac ultrasonography can be divided into TTE and TEE. Although the latter can more clearly show the structure of the heart and more precisely measure heart function, its operation is more complicated, and for MODS patients especially, the risk posed by TEE examination is significantly increased. Therefore, in clinical practice, doctors often choose TTE to examine patients (10). The results of this study support ICU doctors performing TTE examinations on patients. It should be noted that when considering the TTE examination of patients, the indications of TTE need to be fully considered, and not all patients should undergo TTE examination. The indications recommended by the current guidelines mainly include sudden decline in cardiac function, structural changes in the original heart, and suspected acute changes in cardiac structure (such as heart rupture, papillary muscle rupture, paravalvular leakage, heart and large vessel thrombosis, etc.) (23). Therefore, if the MODS does not largely involve the heart or if there is no evidence to suggest changes in the heart structure or thrombosis, active TTE examinations are not recommended. Moreover, study has shown that for most patients, TTE can provide a sufficient amount information for disease identification and assessment (24). Finally, once the patient has indications for TTE, immediate use of TTE is recommended.
Owing to the advances made in medical science and technology, there is a wide array of assessment methods available for use in clinical practice. Ultrasound examination is a noninvasive, convenient, real-time, intuitive, low-cost, and relatively accurate method, and as such, offers a broad range of clinical applications, especially for important organs such as the heart, liver, and kidneys. A detailed examination can provide doctors with the required information for evaluating a given condition and for making clinical decisions. However, an excess of information does not change or improve the accuracy of evaluation, nor does it better inform decision-making. On the contrary, it may prolong treatment time, increase medical costs, waste medical resources, and affect other patients who require timely examination (25,26). Therefore, researchers have paid greater attention to rational selection of inspection methods. The results of this study suggest that, for patients with MODS in the ICU, TTE can be used to assess the changes in these patients’ cardiac structure and function according to their specific conditions, which may provide reasonable and timely treatment based on the examination results. Our results show that timely TTE examination may be associated with the selection of suitable treatment. Heart function is closely related to blood pressure changes, the use of vasoactive drugs, and IV fluid infusion. According to the results of TTE, doctors can more accurately determine the time and dosage of vasoactive drugs and can also avoid the under- or overprovision of IV fluid infusion. This is important because an excess of fluid input can further increase the burden on the heart and lungs, while insufficient fluid input may be an impediment to maintaining blood pressure and peripheral tissue perfusion.
Some limitations of this study should be noted. As mentioned above, the two commonly used examination methods for cardiac ultrasound are TTE and TEE. Although we failed to compare the significance of these two methods for patients with MODS, we did find that TEE has more stringent indications. Therefore, future studies can conduct randomized controlled studies for patients who simultaneously meet the indications of TTE and TEE so as to further explore which method of cardiac ultrasound examination is better for MODS patients. Moreover, as this study was based on the MIMIC-III database, it was a retrospective study of real-world data; a large number of cases were excluded because they did not meet the inclusion criteria, which might have led to the introduction of selection bias.
Acknowledgments
Funding: This study was supported by the Key Projects of Natural Science Foundation of Liaoning Province (Nos. 20180530010 and 2021-MS-328), and the Peking Union Medical College Foundation Rui E (Ruiyi) Emergency Medical Research Fund (No. R2018002).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-717/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-717/coif). All authors report that this work was supported by the Key Projects of Natural Science Foundation of Liaoning Province (Nos. 20180530010 and 2021-MS-328) and the Peking Union Medical College Foundation Rui E (Ruiyi) Emergency Medical Research Fund (No. R2018002). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med 1998;26:1793-800. [Crossref] [PubMed]
- Khwannimit B. Serial evaluation of the MODS, SOFA and LOD scores to predict ICU mortality in mixed critically ill patients. J Med Assoc Thai 2008;91:1336-42. [PubMed]
- Peres Bota D, Melot C, Lopes Ferreira F, et al. The Multiple Organ Dysfunction Score (MODS) versus the Sequential Organ Failure Assessment (SOFA) score in outcome prediction. Intensive Care Med 2002;28:1619-24. [Crossref] [PubMed]
- Gourd NM, Nikitas N. Multiple Organ Dysfunction Syndrome. J Intensive Care Med 2020;35:1564-75. [Crossref] [PubMed]
- Typpo KV, Wong HR, Finley SD, et al. Monitoring Severity of Multiple Organ Dysfunction Syndrome: New Technologies. Pediatr Crit Care Med 2017;18:S24-31. [Crossref] [PubMed]
- Campbell SJ, Bechara R, Islam S. Point-of-Care Ultrasound in the Intensive Care Unit. Clin Chest Med 2018;39:79-97. [Crossref] [PubMed]
- Brown SM, Kasal J. Bedside Ultrasound in the Intensive Care Unit: Where Is the Evidence? Semin Respir Crit Care Med 2015;36:878-89. [Crossref] [PubMed]
- Vieillard-Baron A, Millington SJ, Sanfilippo F, et al. A decade of progress in critical care echocardiography: a narrative review. Intensive Care Med 2019;45:770-88. [Crossref] [PubMed]
- Field LC, Guldan GJ 3rd, Finley AC. Echocardiography in the intensive care unit. Semin Cardiothorac Vasc Anesth 2011;15:25-39. [Crossref] [PubMed]
- Jolobe OMP. Transthoracic echocardiography is a noninvasive alternative to TEE. Am J Emerg Med 2020;38:828-9. [Crossref] [PubMed]
- Teran F, Prats MI, Nelson BP, et al. Focused Transesophageal Echocardiography During Cardiac Arrest Resuscitation: JACC Review Topic of the Week. J Am Coll Cardiol 2020;76:745-54. [Crossref] [PubMed]
- Mayo PH, Narasimhan M, Koenig S. Critical Care Transesophageal Echocardiography. Chest 2015;148:1323-32. [Crossref] [PubMed]
- Nagre AS. Focus-assessed transthoracic echocardiography: Implications in perioperative and intensive care. Ann Card Anaesth 2019;22:302-8. [Crossref] [PubMed]
- Hall SF, Griffiths R. Use and overuse of diagnostic neck ultrasound in Ontario: Retrospective population-based cohort study. Can Fam Physician 2020;66:e62-8. [PubMed]
- Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3:160035. [Crossref] [PubMed]
- Feng M, McSparron JI, Kien DT, et al. Transthoracic echocardiography and mortality in sepsis: analysis of the MIMIC-III database. Intensive Care Med 2018;44:884-92. [Crossref] [PubMed]
- Jiang H, Xu W, Chen W, et al. Value of early critical care transthoracic echocardiography for patients undergoing mechanical ventilation: a retrospective study. BMJ Open 2021;11:e048646. [Crossref] [PubMed]
- Li B, Shi D, Zhu L, et al. The impact of transthoracic echocardiography on the short-term prognosis of elderly patients in the intensive care unit: a retrospective analysis based on the MIMIC-III database. Ann Palliat Med 2021;10:7653-61. [Crossref] [PubMed]
- Fry DE. Multiple organ dysfunction syndrome: past, present and future. Surg Infect (Larchmt) 2000;1:155-61; discussion 161-3. [Crossref] [PubMed]
- Marshall JC. Measuring organ dysfunction. Med Klin Intensivmed Notfmed 2020;115:15-20. [Crossref] [PubMed]
- Cobb JP, Buchman TG, Karl IE, et al. Molecular biology of multiple organ dysfunction syndrome: injury, adaptation, and apoptosis. Surg Infect (Larchmt) 2000;1:207-13; discussion 214-5. [Crossref] [PubMed]
- Savira F, Magaye R, Liew D, et al. Cardiorenal syndrome: Multi-organ dysfunction involving the heart, kidney and vasculature. Br J Pharmacol 2020;177:2906-22. [Crossref] [PubMed]
- Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019;32:1-64. [Crossref] [PubMed]
- Law TK, Bouck Z, Yin XC, et al. Association Between Transthoracic Echocardiography Appropriateness and Echocardiographic Findings. J Am Soc Echocardiogr 2019;32:667-73.e4. [Crossref] [PubMed]
- Morgan DJ, Dhruva SS, Coon ER, et al. 2018 Update on Medical Overuse. JAMA Intern Med 2019;179:240-6. [Crossref] [PubMed]
- Morgan DJ, Dhruva SS, Coon ER, et al. 2019 Update on Medical Overuse: A Review. JAMA Intern Med 2019;179:1568-74. [Crossref] [PubMed]
(English Language Editor: J. Gray)


