
The prognosis and risk factors for capecitabine maintenance treatment in metastatic breast cancer: a retrospective comparative cohort study
Introduction
According to the 2020 cancer statistics report, breast cancer (BC) is the most common malignancy in females worldwide (1). Among patients with BC, 4–6% present with advanced BC (ABC) at diagnosis (2). Moreover, 30–40% of patients with early-stage cancer eventually progress to metastatic BC (MBC) (3). While the survival rate of patients with ABC has gradually improved over the past 30 years, the 5-year survival rate remains less than 20% (4). Currently, MBC is incurable. The main focus of MBC treatment is to optimize treatment modalities to relieve symptoms, improve quality of life, and prolong progression-free survival (PFS).
Maintenance treatment was first introduced for leukaemia and subsequently approved for non-small cell lung cancer and colon cancer. Meta-analyses have shown that prolonging first-line chemotherapy duration can significantly improve overall survival (OS) and PFS (5,6). In December 2017, the Beijing consensus for maintenance therapy in BC recommended prolonging the duration of chemotherapy treatment in patients with ABC who have achieved disease control, including complete response (CR), partial response (PR), and stable disease (SD), after receiving standardized first-line chemotherapy (usually six to eight cycles). For patients who tolerated and benefited from first-line chemotherapy, continuous use of a combination regimen may be considered; however, not all drugs that are effective in combination chemotherapy are suitable for long-term maintenance therapy (7,8). Ideal maintenance medicine should be an effective single drug, well tolerated, suitable for long-term use, and easy to administer, while economic factors should also be considered. Candidate drugs include taxanes, gemcitabine, capecitabine, and vinorelbine (9,10).
Capecitabine, an oral fluorouracil, has demonstrated a curative effect in the treatment of ABC. The ML25241 study showed the efficacy and safety of capecitabine monotherapy following capecitabine-based first-line chemotherapy in small-sample retrospective studies in China (11-14). In addition, the IMELDA study demonstrated that capecitabine significantly improved the efficacy of bevacizumab in maintenance treatment (15). The CBCSG010 trial also showed the benefit of capecitabine in adjuvant maintenance in triple-negative BC (TNBC) (16). In patients with early-stage TNBC who received standard adjuvant treatment, low-dose capecitabine maintenance therapy for 1 year significantly improved disease-free survival (17). However, only few studies have focused on the prognostic factors of capecitabine maintenance treatment. Zhu et al. reported that patients with non-basal-like TNBC could benefit from capecitabine maintenance therapy and CK5/6 and EGFR were biomarkers (18).
This current investigation retrospectively recruited 482 consecutive patients with MBC who obtained clinical benefits from capecitabine-based chemotherapy between January 2011 and August 2019. The effect of capecitabine maintenance therapy was evaluated and analysis of the circulating tumour DNA (ctDNA) was performed to screen for genetic aberrations responsible for poor treatment response. We present the following article in accordance with the STROBE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3828/rc).
Methods
Study design
This was a retrospective cohort study conducted at Hunan Cancer Hospital in China. The study cohort included 482 consecutive patients with MBC who received first-line or late-line (≥2) chemotherapy and obtained clinical benefits (CR, PR, or SD) at Hunan Cancer Hospital, the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University from January 2011 to August 2019. Two hundred and ninety-five received first-line capecitabine-based chemotherapy following MBC diagnosis, and 187 received late-line (≥2) capecitabine-based chemotherapy. Two hundred and fifty-six continued to receive capecitabine maintenance therapy (1,000 mg, BID, 3-week cycle) alone [in TNBC and hormone receptor (HR)-positive/ human epidermal growth factor receptor 2 (HER2)-negative patients] or in combination with anti-HER2 therapy (in HER2-positive patients) (Figure 1). The remaining 226 patients did not receive capecitabine maintenance therapy. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee at Hunan Cancer Hospital, the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University (No. KYJJ19033). Informed consent was taken from all the patients. A retrospective independent radiologic committee was used to confirm the validity of the PFS end point findings.

Patients and baseline clinical features
The inclusion criteria were as follows: (I) patients with measurable metastatic lesions with pathological confirmation; (II) patients with available up-to-date HER2, progesterone receptor (PR), and estrogen receptor (ER) status; (III) patients who received prior capecitabine-based chemotherapy following MBC diagnosis; and (IV) patients with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1. The exclusion criteria were as follows: (I) patients with no updated available ER/PR/HER2/Ki-67 status; (II) patients with no measurable metastatic lesions; (III) patients with an ECOG performance status of 2–4; (IV) patients with heart disease or heart abnormalities, such as cardiac infarction or severe cardiac arrhythmia; and (V) patients with liver or renal function disorders. The baseline clinical features included the age at diagnosis, menopause, neoadjuvant therapy, ER, PR, HER2 status and subtypes, prior treatment lines, and prior capecitabine-based treatment response.
Study treatment group and follow up procedures
The data flow and treatment groups of this retrospective observational study are shown in Figure 1. Among the 482 patients who achieved clinical benefit from capecitabine-based chemotherapy, 295 received first-line capecitabine-based chemotherapy following MBC diagnosis, and 187 received late-line (≥2) capecitabine-based chemotherapy. Image evaluation was performed after every two treatment cycles. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria version 4.0 (13). The capecitabine maintenance dose was 1,000 mg bid (3-week cycle). For patients with grade 3 hand-foot syndrome, the dose was decreased to 650 mg (19).
According to the Response Evaluation Criteria in Solid Tumours1.1 standard (20-22), among patients who benefited from capecitabine-based chemotherapy and achieved a CR, PR, or SD, 256 continued to receive capecitabine maintenance therapy (1,000 mg, BID, 3-week cycle) alone (in TNBC and HR-positive/HER2-negative patients) or in combination with anti-HER2 therapy (in HER2-positive patients) (Figure 1). The remaining 226 patients did not receive capecitabine maintenance therapy. Patients with TNBC did not receive maintenance treatment. Patients who were HR positive received endocrine therapy. Patients with HER2 positivity and HR negativity received anti-HER2 treatment alone. Patients who were HER2 positive and HR positive received endocrine therapy plus anti-HER2 treatment. Since most patients were lost after disease progression, so the OS was not the treatment outcome of this study. Treatment outcome (PFS) was assessed by imaging tools according to RSCIST 1.1 standard during the first two treatment cycles and every 3 weeks thereafter. During the period of maintenance treatment, patients who progressed were switched to late-line therapy.
Image surveillance and ctDNA testing
Baseline imaging and ctDNA evaluations were performed 3–7 days prior to the initiation of capecitabine-based treatment. Image surveillance was performed in all patients after every two treatment cycles. In total, 73 maintenance patients voluntarily underwent commercial ctDNA testing. The experimental protocols for bio-specimen and DNA extraction, ctDNA capture, hybridization, sequencing, and mutation identification have been previously described in detail (23-26). To investigate the significant ctDNA aberrations responsible for a poor or lack of response to capecitabine-based treatment, the R package ‘Complex Heatmap’ was applied to rank the high-risk genetic aberrations in these patients. Genetic aberrations were compared between subgroups. The ctDNA aberration analysis was conducted using R 4.1.2 software. The ctDNA study was performed based on a platform study (ACTDNA, NCT05079074).
Statistical analyses
Categorical variables are summarised as counts (percentages) for analysis of the demographic and clinical variables. Differences in categorical variables between subgroups were compared using the chi-squared test. The Mantel-Haenszel chi-squared test was used when the number of subgroups was greater than two. Quantitative variables are presented as the mean ± standard deviation or the median [interquartile range (IQR)]. The differences in quantitative variables between two subgroups were analyzed using the Student’s t-test.
To evaluate the effect of capecitabine maintenance on PFS and to reduce the potential bias between the maintenance and non-maintenance groups, the propensity score matching (PSM) analysis was applied. Kaplan-Meier curves and a two-sided log-rank test were used to assess the influence of HR/HER2 subtypes on PFS in capecitabine maintenance. Patients who did not progress were censored on the date of their last follow-up. Univariate and multivariate Cox proportional hazards models were used to estimate the treatment effect. The SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and R 3.6.2 (https://www.r-project.org/) software were used to perform statistical analyses. All hypothesis tests were two-sided and conducted at a significance level of 0.05.
Results
Patients and their demographic/clinical features
Of the recruited 482 patients, 256 were sensitive to capecitabine-based chemotherapy and subsequently received capecitabine maintenance therapy. Among the 256 patients who received capecitabine maintenance therapy, 46 patients with TNBC and 130 HR-positive and HER2-negative patients received capecitabine maintenance alone and 80 HER2-positive patients received capecitabine maintenance plus anti-HER2 treatment. In total, 226 patients received endocrine maintenance (100 HR-positive and HER2-negative patients), anti-HER2 treatment (21 HR-negative and HER2-positive patients), anti-HER2 treatment plus endocrine maintenance (31 HR-positive and HER2-positive patients), or no maintenance therapy (74 patients with TNBC) (Figure 1). The median follow-up time was 8.6 months (IQR: 4.5–16.37 months)
Regarding the demographic and clinical features, there were no significant differences in diagnostic age, menopause history, stage at diagnosis, prior neoadjuvant therapy, nor surgical intervention between the maintenance and non-maintenance subgroups (Table 1). However, the molecular status of the biopsies was significantly different between the groups. There was a greater number of HR-positive and HER2-positive patients in the capecitabine maintenance group compared to the non-capecitabine maintenance group. In addition, significantly more patients with the TNBC subtype were detected in the non-capecitabine maintenance group. These findings suggested that more HR-positive and HER2-positive patients received capecitabine maintenance.
Table 1
| Variables | Total (n=482) | Capecitabine maintenance | P value* | |
|---|---|---|---|---|
| Yes (n=256) | No (n=226) | |||
| Age at diagnosis (years)# | 45.03±9.51/45 [39, 51] | 44.66±9.23/45 [38, 51] | 45.46±9.81/45 [39, 52] | 0.36 |
| Menopause, n (%) | 0.81 | |||
| No | 325 (67.43) | 174 (67.97) | 151 (66.81%) | |
| Yes | 157 (32.57) | 82 (32.03) | 75 (33.19%) | |
| Neo-adjuvant therapy, n (%) | 0.26 | |||
| Yes | 98 (20.33) | 57 (22.27) | 41 (18.14) | |
| No | 384 (79.67) | 199 (77.73) | 185 (81.86) | |
| Surgery, n (%) | 0.74 | |||
| Yes | 456 (94.61) | 243 (94.92) | 213 (94.25) | |
| No | 26 (5.39) | 13 (5.08) | 13 (5.75) | |
| ER, n (%) | 0.03 | |||
| Positive | 281 (58.30) | 161 (62.89) | 120 (53.10) | |
| Negative | 201 (41.70) | 95 (37.11) | 106 (46.90) | |
| PR, n (%) | 0.03 | |||
| Positive | 251 (52.07) | 145 (56.64) | 106 (46.90) | |
| Negative | 231 (47.93) | 111 (43.36) | 120 (53.10) | |
| HER2, n (%) | 0.04 | |||
| Positive | 132 (27.39) | 80 (31.25) | 52 (23.01) | |
| Negative | 350 (72.61) | 176 (68.75) | 174 (76.99) | |
| Subtype, n (%) | 0.002 | |||
| Triple negative | 120 (24.90) | 46 (17.97) | 74 (32.74) | |
| HR+/HER2− | 230 (47.72) | 130 (50.78) | 100 (44.25) | |
| HR−/HER2+ | 55 (11.41) | 34 (13.28) | 21 (9.29) | |
| HR+/HER2+ | 77 (15.98) | 46 (17.97) | 31 (13.72) | |
| Prior chemotherapy lines, n (%) | 0.21 | |||
| 1 | 384 (57.40) | 163 (63.67) | 132 (58.41) | |
| 2 | 206 (30.79) | 71 (27.73) | 71 (31.42) | |
| ≥3 | 79 (11.81) | 22 (8.59) | 23 (10.17) | |
| Prior chemotherapy response, n (%) | 0.72 | |||
| CR | 18 (3.13) | 8 (3.13) | 10 (4.42) | |
| PR | 169 (35.06) | 92 (35.94) | 77 (34.07) | |
| SD | 295 (61.20) | 156 (60.94) | 139 (61.50) | |
*, P values were calculated using a Student’s t-test for continuous variables and a chi-square test (Mentel-Haenszel for >2 levels comparison) or Fisher’s exact test (n<5) for categorical variables. Age at diagnosis (years)# represented as the average age at diagnosis with standard deviation and the median age at diagnosis with IQR. MBC, metastatic breast cancer; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; CR, complete response; PR, partial response; SD, stable disease; IQR, interquartile range.
Risk factors for treatment outcomes
For patients who received capecitabine-based chemotherapy and obtained clinical benefits (CR, PR, and SD), the risk factors for subsequent PFS were evaluated using Cox regression analysis. HR-positive and HER2-positive subtypes were significant beneficial factors for PFS [univariate: hazard ratio (HR) =0.50, 95% confidence interval (CI): 0.30–0.86, P=0.01; multivariate: HR =0.48, 95% CI: 0.28–0.82, P=0.007; Table S1]. However, capecitabine maintenance did not show any benefit in HR-positive and HER2-positive patients, and only had a marginal benefit on PFS in patients with TNBC (HR =0.57, 95% CI: 0.28–1.16, P=0.12).
To reduce the potential bias between the capecitabine maintenance and non-maintenance subgroups, PSM was performed. Table S2 shows that the patients were balanced between the capecitabine maintenance and non-maintenance groups after PSM. Patients with HR-positive and HER2-positive MBC still showed a significant protective effect on the PFS (univariate: HR =0.53, 95% CI: 0.30–0.94, P=0.03; multivariate: HR =0.49, 95% CI: 0.27–0.89, P=0.02; Table S3) in the PMS-matched cohort. Capecitabine maintenance still did not show a significant benefit in PFS in the matched cohort.
Clinical risk factors for capecitabine maintenance treatment
In the capecitabine maintenance group, both univariate and multivariate Cox regression analyses showed that first-line therapy and HR-positive/HER2-positive subtypes were significant protective factors for PFS (Table 2). First-line capecitabine-based chemotherapy followed by capecitabine maintenance resulted in a significantly lower risk of progression compared with late-line capecitabine-based chemotherapy followed by capecitabine maintenance (univariate: HR =0.67, 95% CI: 0.46–0.97, P=0.03; multivariate: HR =0.67, 95% CI: 0.45–1.00, P=0.05). HR-positive and HER2-positive subtypes were also favourable clinical factors (univariate: HR =0.34, 95% CI: 0.18–0.65, P=0.001; multivariate: HR =0.33, 95% CI: 0.17–0.63, P=0.0008).
Table 2
| Covariates | Univariate Cox regression | Multivariate Cox regression | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age at diagnosis# | 1.002 (0.983, 1.022) | 0.82 | 1.016 (0.984, 1.048) | 0.33 | |
| Menopause | |||||
| No | Ref | Ref | |||
| Yes | 0.91 (0.61,1.35) | 0.63 | 0.75 (0.41,1.40) | 0.37 | |
| Neo-adjuvant therapy | |||||
| Yes | Ref | Ref | |||
| No | 0.90 (0.58, 1.41) | 0.65 | 0.94 (0.59, 1.51) | 0.80 | |
| Surgery | |||||
| Yes | Ref | Ref | |||
| No | 0.79 (0.35, 1.79) | 0.57 | 0.93 (0.40, 2.20) | 0.87 | |
| ER | |||||
| Positive | Ref | Ref | |||
| Negative | 0.85 (0.59, 1.22) | 0.38 | 1.03 (0.65, 1.63) | 0.91 | |
| PR | |||||
| Positive | Ref | Ref | |||
| Negative | 0.65 (0.45, 0.92) | 0.02 | 0.53 (0.34, 0.84) | 0.007 | |
| HER2 | |||||
| Positive | Ref | Ref | |||
| Negative | 0.70 (0.47, 1.04) | 0.08 | 0.65 (0.43, 1.00) | 0.05 | |
| Subtype | |||||
| Triple negative | Ref | Ref | |||
| HR+/HER2− | 0.59 (0.37, 0.92) | 0.02 | 0.51 (0.32, 0.82) | 0.005 | |
| HR−/HER2+ | 0.66 (0.37, 1.17) | 0.16 | 0.64 (0.35, 1.20) | 0.16 | |
| HR+/HER2+ | 0.34 (0.18, 0.65) | 0.001 | 0.33 (0.17, 0.63) | 0.0008 | |
| Prior chemotherapy lines | |||||
| 1 | 0.67 (0.46, 0.97) | 0.03 | 0.67 (0.45, 1.00) | 0.05 | |
| ≥2 | Ref | Ref | |||
| Prior chemotherapy response | |||||
| CR | Ref | Ref | |||
| PR | 0.86 (0.59, 1.25) | 0.42 | 0.90 (0.61, 1.31) | 0.58 | |
| SD | 0.41 (0.10, 1.67) | 0.21 | 0.51 (0.12, 2.20) | 0.37 | |
Age at diagnosis (years)# represented as the average age at diagnosis with standard deviation and the median age at diagnosis with IQR. PFS, progression-free survival; MBC, metastatic breast cancer; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; CR, complete response; PR, partial response; SD, stable disease; IQR, interquartile range.
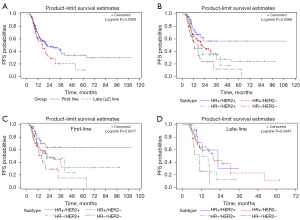
Survival analysis was performed for patients who received different lines of capecitabine-based chemotherapy and for those with different HR/HER2 subtypes. The results are shown in Figure 2. Generally, patients who received first-line capecitabine-based chemotherapy had a significantly longer PFS than those who received late-line (≥2) chemotherapy (log-rank P=0.0309; Figure 2A). In addition, in the capecitabine maintenance group, HR-positive and HER2-positive patients showed the greatest benefit from capecitabine maintenance, whereas patients with TNBC had the poorest PFS (log-rank P=0.0068; Figure 2B). This difference was significant in both the first-line and late-line capecitabine-based chemotherapy groups, followed by the capecitabine maintenance group (Figure 2C,2D).
Genetic risk factors for capecitabine maintenance treatment
In addition to pathological HR/HER2 subtypes and treatment timing, genetic aberrations can also influence the treatment response and outcome in capecitabine maintenance MBC patients (23). To evaluate influential genetic aberrations, 57 patients in the capecitabine maintenance group underwent commercial ctDNA testing. As shown in Figure 3A, the incidence rates of PIK3CA and TP53 aberrations in the capecitabine maintenance group were 39% and 30%, respectively.
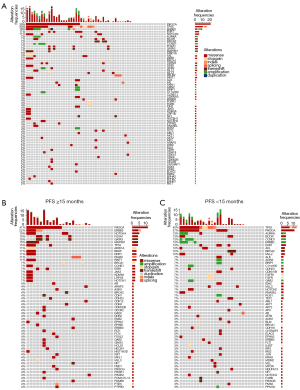
To further investigate the genetic aberrations that may influence the treatment outcome of capecitabine maintenance, we divided the capecitabine maintenance group into two subgroups: the long-PFS subgroup (PFS ≥15 months) and the short-PFS subgroup (PFS <15 months). As shown in Figure 3B,3C, 13/30 (43%) patients in the short-PFS subgroup had TP53 aberrations, whereas only 4/27 (15%) patients in the long-PFS subgroup had TP53 aberrations (chi-squared test, P=0.04). These results indicated that the incidence of TP53 aberrations correlates with patient resistance to capecitabine maintenance. In addition, in comparison with patients resistant to prior capecitabine-based chemotherapy who had the highest TP53 aberration rate (63%, Figure S1), the short PFS subgroup of the capecitabine maintenance group had a relatively lower TP53 aberration rate (43%), and patients with a long PFS in the capecitabine maintenance group had the lowest TP53 aberrant rate (Mantel-Haenszelchi-squared test P<0.0001, Table S4), suggesting that TP53 aberration is negatively correlated with patient response to capecitabine-based chemotherapy and maintenance.
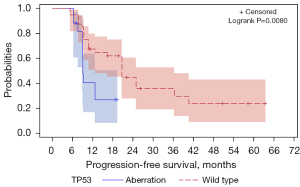
In addition to TP53 aberrations, PIK3CA aberrations may also play a role in the effect of capecitabine maintenance. Figure S1 shows that patients who had progressive disease (PD) in capecitabine-based chemotherapy displayed a similar PIK3CA aberration rate to those who were sensitive (37% vs. 39%, Figure 3A). Additionally, patients in the short-PFS subgroup also had a similar rate of PIK3CA aberration to those in the long-PFS subgroup (37% vs. 41%, Figure 3B,3C). Survival analysis showed TP53 alterations to be a significant risk factor for poor PFS (log-rank test, P=0.008, Figure 4). However, PIK3CA aberrations were not a significant risk factor for poor PFS in the capecitabine maintenance group.
Side effects
In general, the survival benefit and treatment-related toxicity of drug therapy must be balanced. According to the literature (13,27,28), capecitabine is a suitable agent for maintenance treatment. Herein, 60–80% of patients in the maintenance group experienced neutropenia and had a decreased white blood cell count, but none were grade 3 or 4 events. The incidence rates of adverse skin reactions in the maintenance and non-maintenance groups were 1.17% and 0.44%, respectively. Compared to the non-maintenance group, the maintenance group showed a higher incidence of hand-foot syndrome (9.07% vs. 0%; Table S5). Only 3.52% of patients were grade 3. Barely intolerable side effects were detected during capecitabine maintenance treatment. Only 1.56% and 2.73% of patients in the maintenance group had tolerable neurotoxicity and nausea, respectively. These results suggested that capecitabine maintenance alone or in conjunction with anti-HER2 agents is well tolerated.
Discussion
Clinical guidelines state that after first-line treatment, the treatment should continue until the disease progresses or the toxicity is intolerable. The use of a single drug in an effective first-line maintenance regimen not only avoids the toxic effects of combined regimen maintenance but also reduces the risk of cross-resistance. Capecitabine is the standard treatment for MBC following anthracycline or taxane treatment failure and is also an ideal maintenance treatment for BC. Capecitabine maintenance therapy has been shown to improve PFS in ABC (11), and when used for metronomic chemotherapy, it can improve survival in patients with BC (29). Our hospital also concurs with the administration of capecitabine in patients with MBC. The incidence rates of severe adverse events in the liver, kidney, skin, gastrointestinal tract, and haematological and nervous systems were low. The occurrence of hand-foot syndrome can be reduced and controlled by prevention and early intervention.
In comparison with other immunohistochemical (IHC) 4 subtypes, HR-positive/HER2-positive patients had the most favourable PFS. This demonstrated the efficacy of capecitabine maintenance in HR-positive/HER2-positive patients, but also implied a synergic action of capecitabine and anti-HER2 agents. Other combinations have been identified in clinical trials. The IMELDA and GINECOA-TaXel trials suggested that capecitabine plus bevacizumab maintenance significantly improved PFS and OS following first-line bevacizumab plus taxane and capecitabine chemotherapy (15,28). In patients with MBC with HR positivity and HER2 negativity, sequential endocrine therapy in combination is the preferred therapeutic option, with the exception of patients with visceral crisis (30,31). Among HR-positive and HER2-positive patients, HER2-targeted therapy plus an aromatase inhibitor could be effective for those who are unsuitable for chemotherapy (30). In comparison with other maintenance strategies, capecitabine maintenance showed comparable performance.
After prior chemotherapy, the optimization of the maintenance strategy should involve several factors, including endocrine-sensitive status, prior use of drugs, and other prognostic risk factors, such as genetic aberrations. Previous reports have shown that TP53 mutations in BC can induce resistance to chemotherapy and systematic therapies (32-36). Our current study suggested that TP53 aberrations were most prevalent in patients with PD during capecitabine-based chemotherapy (63%, Figure S1). In the short-PFS subgroup, TP53 aberrations were moderately prevalent (43%, Figure 3C). In the long-PFS subgroup, only 15% of the patients had TP53 aberrations (Figure 3B). Survival analysis showed that TP53 was significantly associated with poor PFS in the capecitabine maintenance group (Figure S2).
The present study clarified the clinical and genetic factors associated with the treatment outcomes of capecitabine maintenance. Prior first-line chemotherapy and the HR-positive/HER2-positive subtypes were significant beneficial factors, whereas TNBC and late-line chemotherapy were detrimental factors. TP53 alterations are significant genetic issues related to drug resistance.
This study had several limitations. There were relatively small numbers of patients in each subgroup. Some patients who responded to first- or second-line capecitabine but who did not continue onto maintenance therapy, were recruited in this study. This was not a selection bias. Consecutive patients who received capecitabine-based chemotherapy at the center were recruited and some did not continue capecitabine therapy because they selected to use other maintenance drugs, such as tamoxifen or trastuzumab. Some patients with TNBC were reluctant to receive continuous chemotherapy maintenance because of their internal resistance and conservative ideas.
Acknowledgments
Funding: This study was supported by the Hunan Nature and Science Foundation (Nos. 2020JJ8064 and 2019JJ50360); the Hunan Health Commission Program (Nos. B2019089, and C2019070); the Changsha City Technology Program (Nos. kq1901076, kq2004137, and kq2004125); and the National Science Foundation of China (No. 61972147).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3828/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3828/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3828/coif).The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee at Hunan Cancer Hospital, the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University (No. KYJJ19033). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer 2021; Epub ahead of print. [Crossref] [PubMed]
- Cardoso F, Fallowfield L, Costa A, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2011;22:vi25-30. [Crossref] [PubMed]
- Huober J, Thürlimann B. The Role of Combination Chemotherapy in the Treatment of Patients with Metastatic Breast Cancer. Breast Care (Basel) 2009;4:367-72. [Crossref] [PubMed]
- Sundquist M, Brudin L, Tejler G. Improved survival in metastatic breast cancer 1985-2016. Breast 2017;31:46-50. [Crossref] [PubMed]
- Gennari A, Stockler M, Puntoni M, et al. Duration of chemotherapy for metastatic breast cancer: a systematic review and meta-analysis of randomized clinical trials. J Clin Oncol 2011;29:2144-9. [Crossref] [PubMed]
- Coates A, Gebski V, Bishop JF, et al. Improving the quality of life during chemotherapy for advanced breast cancer. A comparison of intermittent and continuous treatment strategies. N Engl J Med 1987;317:1490-5. [Crossref] [PubMed]
- Gennari A, Amadori D, De Lena M, et al. Lack of benefit of maintenance paclitaxel in first-line chemotherapy in metastatic breast cancer. J Clin Oncol 2006;24:3912-8. [Crossref] [PubMed]
- Alba E, Ruiz-Borrego M, Margelí M, et al. Maintenance treatment with pegylated liposomal doxorubicin versus observation following induction chemotherapy for metastatic breast cancer: GEICAM 2001-01 study. Breast Cancer Res Treat 2010;122:169-76. [Crossref] [PubMed]
- Park YH, Jung KH, Im SA, et al. Phase III, multicenter, randomized trial of maintenance chemotherapy versus observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol 2013;31:1732-9. [Crossref] [PubMed]
- Cinieri S, Chan A, Altundag K, et al. Final Results of the Randomized Phase II NorCap-CA223 Trial Comparing First-Line All-Oral Versus Taxane-Based Chemotherapy for HER2-Negative Metastatic Breast Cancer. Clin Breast Cancer 2017;17:91-99.e1. [Crossref] [PubMed]
- Wang J, Xu B, Yuan P, et al. Capecitabine combined with docetaxel versus vinorelbine followed by capecitabine maintenance medication for first-line treatment of patients with advanced breast cancer: Phase 3 randomized trial. Cancer 2015;121:3412-21. [Crossref] [PubMed]
- Dong G, Jia Y, Wang X, et al. The comparison of maintenance treatment with capecitabine (CMT) and non-maintenance treatment with capecitabine (non-CMT) in patients with metastatic breast cancer. Int J Clin Exp Med 2015;8:8283-7. [PubMed]
- Liang X, Di L, Song G, et al. Capecitabine maintenance therapy for XT chemotherapy-sensitive patients with metastatic triple-negative breast cancer. Chin J Cancer Res 2014;26:550-7. [PubMed]
- Lv H, Yan M, Zhang M, et al. Efficacy of capecitabine-based combination therapy and single-agent capecitabine maintenance therapy in patients with metastatic breast cancer. Chin J Cancer Res 2014;26:692-7. [PubMed]
- Gligorov J, Doval D, Bines J, et al. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1351-60. [Crossref] [PubMed]
- Li J, Yu K, Pang D, et al. Adjuvant Capecitabine With Docetaxel and Cyclophosphamide Plus Epirubicin for Triple-Negative Breast Cancer (CBCSG010): An Open-Label, Randomized, Multicenter, Phase III Trial. J Clin Oncol 2020;38:1774-84. [Crossref] [PubMed]
- Wang X, Wang SS, Huang H, et al. Effect of Capecitabine Maintenance Therapy Using Lower Dosage and Higher Frequency vs Observation on Disease-Free Survival Among Patients With Early-Stage Triple-Negative Breast Cancer Who Had Received Standard Treatment: The SYSUCC-001 Randomized Clinical Trial. JAMA 2021;325:50-8. [Crossref] [PubMed]
- Zhu Y, Li K, Zhang J, et al. The prognostic and predictive significance of cytokeratin 5/6 and epidermal growth factor receptor in metastatic triple-negative breast cancer treated with maintenance capecitabine. Transl Cancer Res 2021;10:1193-203. [Crossref] [PubMed]
- Qin D, Wang J, Le A, et al. Traumatic Brain Injury: Ultrastructural Features in Neuronal Ferroptosis, Glial Cell Activation and Polarization, and Blood-Brain Barrier Breakdown. Cells 2021;10:1009. [Crossref] [PubMed]
- Litière S, de Vries EGE, Seymour L, et al. The components of progression as explanatory variables for overall survival in the Response Evaluation Criteria in Solid Tumours 1.1 database. Eur J Cancer 2014;50:1847-53. [Crossref] [PubMed]
- Mandrekar SJ, An MW, Meyers J, et al. Evaluation of alternate categorical tumor metrics and cut points for response categorization using the RECIST 1.1 data warehouse. J Clin Oncol 2014;32:841-50. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Hu ZY, Xie N, Tian C, et al. Identifying Circulating Tumor DNA Mutation Profiles in Metastatic Breast Cancer Patients with Multiline Resistance. EBioMedicine 2018;32:111-8. [Crossref] [PubMed]
- Yang X, Chu Y, Zhang R, et al. Technical Validation of a Next-Generation Sequencing Assay for Detecting Clinically Relevant Levels of Breast Cancer-Related Single-Nucleotide Variants and Copy Number Variants Using Simulated Cell-Free DNA. J Mol Diagn 2017;19:525-36. [Crossref] [PubMed]
- Phallen J, Sausen M, Adleff V, et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med 2017;9:eaan2415. [Crossref] [PubMed]
- Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010;26:589-95. [Crossref] [PubMed]
- Ciruelos E, Pérez-García JM, Gavilá J, et al. Maintenance Therapy in HER2-Negative Metastatic Breast Cancer: A New Approach for an Old Concept. Clin Drug Investig 2019;39:595-606. [Crossref] [PubMed]
- Ferrero JM, Hardy-Bessard AC, Capitain O, et al. Weekly paclitaxel, capecitabine, and bevacizumab with maintenance capecitabine and bevacizumab as first-line therapy for triple-negative, metastatic, or locally advanced breast cancer: Results from the GINECO A-TaXel phase 2 study. Cancer 2016;122:3119-26. [Crossref] [PubMed]
- Weadick C, Larsson K, O'Reilly S, et al. Efficacy and tolerability of metronomic chemotherapy in patients with metastatic breast cancer - an international experience in West Sweden and in the South of Ireland. Cancer Treat Res Commun 2020;25:100237. [Crossref] [PubMed]
- Rugo HS, Rumble RB, Macrae E, et al. Endocrine Therapy for Hormone Receptor-Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 2016;34:3069-103. [Crossref] [PubMed]
- Sutherland S, Miles D, Makris A. Use of maintenance endocrine therapy after chemotherapy in metastatic breast cancer. Eur J Cancer 2016;69:216-22. [Crossref] [PubMed]
- Andersson J, Larsson L, Klaar S, et al. Worse survival for TP53 (p53)-mutated breast cancer patients receiving adjuvant CMF. Ann Oncol 2005;16:743-8. [Crossref] [PubMed]
- Rahko E, Blanco G, Soini Y, et al. A mutant TP53 gene status is associated with a poor prognosis and anthracycline-resistance in breast cancer patients. Eur J Cancer 2003;39:447-53. [Crossref] [PubMed]
- Mo RJ, Han ZD, Liang YK, et al. Expression of PD-L1 in tumor-associated nerves correlates with reduced CD8+ tumor-associated lymphocytes and poor prognosis in prostate cancer. Int J Cancer 2019;144:3099-110. [Crossref] [PubMed]
- Berns EM, Foekens JA, Vossen R, et al. Complete sequencing of TP53 predicts poor response to systemic therapy of advanced breast cancer. Cancer Res 2000;60:2155-62. [PubMed]
- Gellert P, Segal CV, Gao Q, et al. Impact of mutational profiles on response of primary oestrogen receptor-positive breast cancers to oestrogen deprivation. Nat Commun 2016;7:13294. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


