
Melatonin attenuates acute and chronic itch in mice: the antioxidant and anti-inflammatory effects of melatonin receptors
Introduction
Pruritus (itch) is an unpleasant feeling that results in scratching (1-4). Chronic pruritus is defined as an itching that lasts for >6 weeks (5), and is a common symptom of various dermatologic diseases, such as psoriasis, atopic dermatitis, and allergic contact dermatitis, and systemic diseases, such as cholestasis, and diabetes (6). Chronic itch causes serious skin damage, makes sleeping difficult, and affects patients’ quality of life.
Antihistamines are commonly prescribed to treat itch, but several types of itch are resistant to antihistamines. There are currently few effective medications for chronic itch. Melatonin is a pluripotent molecule with several functions (7). It is secreted by the pineal gland and enters the cerebrospinal fluid and circulatory system where it exhibits a circadian rhythm (7). It is also produced by other tissues, such as the placenta, ovary, testes, skin, and lymphocytes (7). As a well-known mitochondrial targeted antioxidant, melatonin contributes to neuronal protection, circadian rhythms (8), and pain modulation. Melatonin also has an anti-inflammatory effect (9,10). Deteriorations in melatonin levels have been detected in patients with atopic dermatitis (AD), and the association with sleep disorders was revealed in patients with AD (11,12). In addition, melatonin supplementation had beneficial effects on disease severity, sleep-onset latency among patients with AD (11,13).
However, the potential effects and mechanisms of melatonin on acute and chronic itch are unclear. The current study sought to demonstrate that melatonin reduces acute and chronic itching, via melatonin receptors, and its antioxidant, and anti-inflammatory effects. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3804/rc).
Methods
Animals
We purchased all male Institute of Cancer Research (ICR) mice (6–8 weeks of age) from the Shanghai Laboratory Animal Company. All the animals were kept in 12-hour light/dark cycles. Ample food and water were available as needed, and a standard room temperature (22±2 ℃) and a humidity level of 60%±80% were maintained. Animal experiments were performed under a project license (No. 202101L0832) granted by institutional ethics board of Soochow University. All experiments were performed in strict accordance with Guidelines for Laboratory Animal Management issued by Soochow University. Before the study, a protocol was prepared but not registered.
A Power calculation (P<0.05) was conducted for every experiment to ensure that the observed effects were real.
The experimental units were randomly assigned to control and treatment groups using an allocation concealment method. During the experiment, no animals (or experimental units) were excluded, and no data were excluded from the analysis. There were no humane endpoints in this study.
Mouse model of acute itch
As described previously, each mouse’s nape fur was shaved 2 days before the experiments (14). Each mouse was placed in a small plastic chamber on the day of the behavior testing for more than 30 min on a mesh. Under brief isoflurane anesthesia, the mice received intradermal (i.d) injections of compound 48/80 (C48/80) (100 µg) or chloroquine (CQ) (200 µg) in the nape of the neck. A 30-min time lapse recording (Sony HDR-CX610) was made immediately after the i.d injection of the mice. Mouse scratch bouts were recorded. A scratch bout was defined as a mouse lifting its hind paw to scratch the shaved area. Based on an offline playback of the video, the scratching behavior was double-blind quantified.
Imiquimod-induced psoriatic itch in mice
As described previously (15), the hair on the neck of each mouse was shaved with both a clipper and a shaver 3 days before treatment. Each mouse was treated daily for 7 consecutive days by applying 62.5-mg Aldara cream [5% imiquimod (IMQ); Meda Pharmaceuticals] to the shaved back skin (2.52 cm2). The mice in the control group were treated with Vaseline Lanette cream (Fargo). Before the next day of treatment, the spontaneous scratching behavior was quantified based on a 1-hour video recording. The mice were placed in individual plastic chambers for 1 day before being observed. Bouts of scratching were then recorded blindly for 1 hour by the researchers.
Acetone-ether-water (AEW)-induced mouse model of chronic dry-skin itch
At least 3 days before starting AEW treatment, the nape hair of each mouse was shaved. We applied an acetone/ether (1:1) mixture to each mouse’s nape for 30 sec, followed by water for 15 sec. The AEW treatment was administered for 5 days, twice daily, at 9:00 and 17:00 (16). In the control group, cotton was soaked in distilled water and laid on the shaved area for 45 s. The quantification method for the spontaneous scratching behavior was the same as that for the IMQ mouse model.
Alloknesis assays
Briefly, the alloknesis score was identified by counting the number of scratching responses evoked by 0.07 g of von Frey filament, which was stimulated 10 times at intervals of 15 secs in the mice.
Drugs and administration
C48/80 (Cat#C2313), chloroquine (CQ, Cat#C6628), and melatonin (Cat#M5250) were obtained from Sigma-Aldrich (St. Louis, MO, USA).Melatonin receptor type 1 and 2 (MT1/MT2) antagonist Luzindole (Cat#0877/10) and MT2 antagonist 4-P-PDOT (Cat#1034/10) were obtained from Tocris (Bristol, UK). Sirt1 antagonist EX 527 (Cat#S1541) was obtained from Selleck (Shanghai, China). If there were no special instructions, sterile saline was used to dissolve the reagents. An i.d injection of C48/80 (100 mg) and CQ (200 mg) was administered into the nape of each mouse’s neck to induce scratching 30 min after the intraperitoneal (i.p) injection of melatonin. For the psoriatic mice, an i.p injection of melatonin was administered after IMG treatment and at 3, 5, and 7 days, and the mice were immediately placed in plastic chambers for 1 hour and their scratching behavior was observed. The experimental process for the AEW mouse model was the same as that for the psoriatic mouse model.
The measurement of intracellular ROS in ND7-23 cells
2,7-dihydrofluorescein diacetate (DCFH-DA) (Sigma-Aldrich, St. Louis, MO, USA) was used to measure intracellular reactive oxygen species (ROS) levels. Briefly, the ND7-23 cells were cultured in 6-well plates and were seeded at least 12 hours The ND7-23 cells were treated with compound 48/80 (C48/80) or CQ and melatonin for 30 min, and the medium was then substituted with 1 mL of DCFH-DA (25 mM), and the cells were incubated for another 30 min with cold phosphate buffered solution (PBS). In the quantitative analysis, after being treated with the reagents and fluorescence probe, the ND7-23 cells were collected and suspended in 500 µL of PBS for the flow cytometry (FC500, Beckman Coulter, USA) analysis. Median fluorescence intensity (MFI) was measured and analyzed using Cxp (FC500; Beckman Coulter).
Quantitative RT-PCR
In accordance with the manufacturer’s instructions, total ribonucleic acid (RNA) was extracted using Trizol Reagent (Invitrogen, Carlsbad, California). To synthesize complementary deoxyribonucleic acid (cDNA) from 1 ng of total RNA, we used the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, USA). On Applied Biosystems’ Opticon real-time polymerase chain reaction (RT-PCR) detection system (Grand Island, NY), we performed a RT-PCR with the SYBR Green PCR Master Mix (Biotium, Hayward, CA). An estimation of the relative fold of differences expression was made using the 2(-ΔΔ C(t)) method after normalization to Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. By Genewiz, the following below primers were synthesized: Il17a (F: 5'-TTTTCAGCAAGGAATGTGGA-3', R: 5'-TTCATTGTGGAGGGCAGAC-3'), Il22 (F: 5'-TTTCCTGACCAAACTCAGCA-3', R: 5'-CTGGATGTTCTGGTCGTCAC-3'), Il1rl1 (F: TGACTGTCTGGCCCTGAACCT, R: 5'-TCTCTCCAGAACAGAGCAACCTCAA-3'), Il33 (F: 5'-CTGCCTATCCACGGGATTCT-3', R: 5'-ACCGTCGCCTGATTGACTTG-3'), Il31 (F: 5'-CTCCAGAGACCACAGGCAAAG-3', R: 5'-GCCAGCCAGGTTCCTATACAG3-'), Il31ra (F: 5'-AACTGTGACTGCTCCAACTTTGC-3', R: 5'-TATTCCAGCGTTCACACCCAG-3'), Tnf (F: 5'-AGCCGATGGGTTGTACCTTG-3', R: 5'-TTGGGCAGATTGACCTCAGC-3') Cxcl1 (F: 5'-GGCGCCTATCGCCAATG-3', R: 5'-CTGGATGTTCTTGAGGTGAATCC-3'), Mtnr1a (F: 5'-TCAGCGTACACGATAGCAGT-3', R: 5'-TCAGTTTGGGCTTGTTGTCG3'), Mtnr1b (F: 5'-TGATGGGCCTGAGTGTCATT-3', R: 5'-ACGAGGCTGATGTAGATGGG-3').
Histology
We fixed the skin in 4% paraformaldehyde, inserted it in paraffin, and cut it into 10-mm sections. Hematoxylin and eosin (H&E) staining was performed as per standard procedures. The epidermis thickness of each skin sample was measured.
Calcium imaging
Dorsal root ganglia (DRG) from C1–C8 spinal levels were gathered in cold Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 (DMEM/F-12) media, and DRG was digested with collagenase type D (0.2%)/trypsin (0.125%) sequentially at 37 ℃ for 30 min. To plate the neurons on glass coverslips coated with poly-D-lysine (0.5 mg/mL), the neurons were spun at 1,000 ×g and resuspended in DMEM/F-12 media. Before imaging, DRG were cultured with neurobasal media supplemented with 2% B27 (Cat#17504044) and incubated at 37 ℃ for 4 hours. To monitor changes in intracellular calcium ion (Ca2+) levels, the cells were loaded onto a Fluo 4-AM (Cat#F14201) (DRG neurons) for 30 min in the dark at 37 ℃ in calcium imaging buffer (CIB; 5.6 mM of potassium chloride (KCl), 130 mM of sodium chloride (NaCl), 1.2 mM of magnesium chloride (MgCl2), 2.6 mM of Calcium Chloride (CaCl2), 10 mM of Hepes, and 5.6 mM of glucose at pH 7.4). Fluo4 fluorescence intensity was detected with Fluo 4-AM, and the emission at 502 nm was monitored from excitation at 494 nm. CBI was perfused into the imaging chamber for flow for the baseline period was established. Analyte (1 mL) was perfused at a flow rate of 30 s/mL. The response was monitored at 0.5-s intervals for an additional 3,000 s. We detected the effect of melatonin on calcium influx induced by CQ and 48/80, respectively.
ELISAs
Circadian-rhythm experiments were performed on the adult ICR mice, who were kept in normal light from 8 am to 8 pm, and complete darkness from 8 pm to 8 am. After 2 weeks, the mice were randomly divided into the following 5 groups: the vehicle group, the IMQ group, the IMQ + melatonin group, the AEW group, and the AEW + melatonin group. Blood was taken from the eyelid at 6:00 AM 1 week after the mouse model was built, and then taken every 6 hours until 6:00 AM the next day. The enzyme-linked immunoassay (ELISA) kit (ab285251) was used to detect the melatonin content in the serum.
Statistical analysis
Graph Prism 6 (Graph Pad, La Jolla, CA) was used to analyze the data. All the data are expressed as the mean ± standard error of the mean. An unpaired Student’s t-test was used to compare two groups. A one-way analysis of variance (ANOVA) followed by a post-hoc Dunnett test was used for multiple comparisons. Statistical significance was defined as a P value <0.05.
Results
Melatonin decreased acute itch induced by C48/80 and CQ in mice
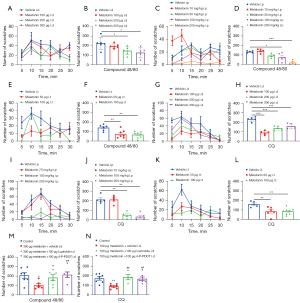
We first discovered that the effect of C48/80 (100 µg)-induced scratching was inhibited by the i.p injection of melatonin (10, 30, 100, and 200 mg/kg) in a dose-dependent manner (see Figure 1A,1B), the i.d co-injection of melatonin (100, 200, and 300 µg) (see Figure 1C,1D), and the intrathecal (i.t) injection of melatonin (50 and 100 µg) (see Figure 1E,1F). We next demonstrated that the effect of CQ (200 µg, i.d.)-induced scratching was attenuated by the i.p injection of melatonin (75, 100, and 200 mg/kg) in a dose-dependent manner (see Figure 1G,1H), the i.d. co-injection of melatonin (100, 200, and 300 µg) (see Figure 1I,1J), and the i.t injection of melatonin (50 and 100 µg) in mice (see Figure 1K,1L). Subsequently, we co-administered the G protein-coupled receptor (GPCR) melatonin receptor MT2 antagonist 4-P-PDOT or non-specific melatonin receptor antagonist luzindole before the i.d co-injection of melatonin and C48/80 or CQ. The results demonstrated that the melatonin-mediated antipruritic effects were abolished by the pretreatment of 4-P-PDOT (100 µg) and luzindole (100 µg) in mice (see Figure 1M,1N).
Melatonin inhibited ROS induced by C48/80 and CQ in ND7-23 cells
Flow cytometry showed that the incubation of C48/80 and CQ substantially increased intracellular ROS expression levels as reflected by increased Dichloro-dihydro-fluorescein diacetate (DCFH-DA) fluorescence intensity in comparison to the control (see Figure 2A-2D). When the ND7-23 cells were exposed to C48/80 (10 µg/mL), the MFI was increased compared to the control, and this effect was attenuated by the pretreatment of melatonin (500 µM; see Figure 2A,2B). Additionally, when ND7-23 was exposed to CQ (1 mM), the MFI values of DCFH-DA were increased relative to the control, and this effect was attenuated by the pretreatment of melatonin (100 µM; see Figure 2C,2D).
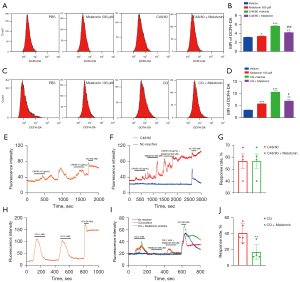
Melatonin inhibited Ca2+ mobilization induced by CQ in primary DRG neurons
Next, we performed calcium imaging experiments using the Fluo4 as a Ca2+ indicator. In the primary cultured DRG neurons, perfusion of C48/80 (10 µg/mL) activated 56.7% of neurons, while melatonin (500 µM) had no obvious inhibitory effect (see Figure 2E-2G). Conversely, the perfusion of melatonin (500 µM) reduced the response rate of CQ (1 mM) (from 40.7% of neurons to 16.9% of neurons) (see Figure 2H-2J).
Melatonin attenuated chronic itch-induced psoriasis or dry skin in mice
Subsequently, systemic therapy with melatonin (50 mg/kg, i.p) considerably attenuated the spontaneous itch and mechanical itch after 5% IMG (IMQ) cream treatment in mice (see Figure 3A,3B). Systemic therapy with melatonin (50 mg/kg, i.p) attenuated spontaneous itch and mechanical itch after AEW treatment in mice (see Figure 3C,3D). H&E staining showed that the epidermis thickness of the involved skin was increased in mice with dry skin and psoriatic skin, while melatonin treatment remarkably reduced the epidermis thickness of the dry-skin and psoriatic mice (see Figure 3E-3H). The ELISA analysis confirmed that systemic treatment with melatonin (50 mg/kg, i.p) significantly enhanced serum melatonin levels in the dry-skin and psoriatic mice (see Figure 3I,3J). The co-administration of the Sirt1 inhibitor EX527 and melatonin abolished the anti-itch effects of melatonin in the chronic itch mice (see Figure 3K,3L).

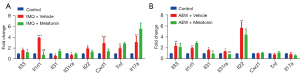
Melatonin attenuated the chronic itch-induced upregulation of pro-inflammatory cytokine expression levels in the skin in mice
Finally, we investigated several cytokines and chemokines in the skin by quantitative RT-PCR. We found that the messenger RNA (mRNA) expression of Interleukin-33 (Il33), IL-1 receptor-like 1 (Il1rl1), interleukin 22 (Il22), chemokine (C-X-C motif) ligand 1(Cxcl1), tumor necrosis factor (Tnf), and interluekin-17A (Il17a) were considerably increased in the skin of psoriatic mice (see Figure 4A), and the mRNA expression of Il33, Il1rl1, interleukin-31 (Il31), interleukin-31 receptor A (Il31ra), and Il22 were considerably increased in the dry-skin mice (see Figure 4B). The mRNA expression of Il33 and Il1rl1 in the skin was suppressed by melatonin (50 mg/kg) in the psoriatic mice (see Figure 4A), while mRNA expression of Il31 and Il31ra in the dry-skin mice was suppressed by melatonin (50 mg/kg) (see Figure 4B).
Discussion
Itching signals are transmitted from the trigeminal ganglia and DRG to the spinal cord dorsal horn, next to the brain (17). Acute itch is traditionally categorized as either histamine-dependent or histamine-independent. Histamine, released by mast cells and keratinocytes, activates PLC3 and TRPV1 receptors on free nerve terminals of the skin, causing itching. Meanwhile, histamine-independent itch can be triggered by multiple mechanisms. An antimalaria drug, CQ, induces histamine-independent itch by binding to Mas-related G proteins (MrgprA3) and activating TRPA1. Peripheral TRPV1-expressing C-fibers are required for both histamine-dependent and histamine-independent itch (17,18). According to our study (unpublished data), melatonin reverse the expression of TRPV1 in the psoriasis model, but not affect the expression in dry-skin mouse models. Acute itch is also caused by other receptors and/or channels, such as TRPV4, which plays a role in histamine- and serotonin-induced itch (19,20), Cav3.2 (21), which plays a role in H2S-related itch, and TRPC3, which plays a role in CQ-induced itch (22). In peripheral neurons, loss of ion channel PIEZO1 function greatly reduces both acute and chronic scratching behaviours (23). These receptors and/or channels are all potential targets of melatonin.
The majority of the biological functions of melatonin are mediated via its 2 GPCR receptors; that is, MT1 and MT2 (24). MT1 is localized mainly in glial cells, while MT2 is mainly expressed in DRG neurons (25). In our study, we found that the systemic or local administration of melatonin reduced acute pruritus in mice. Additionally, the anti- pruritus effects caused by the local application of melatonin were abolished by luzindole and 4-P-PDOT, which suggests that MT2 may be involved in melatonin-induced anti- pruritus effects.
Previously, a mouse model showed that oxidants induce histamine-independent pruritus by activating the transient receptor potential ankyrin 1 (TRPA1) (26), and antioxidants [such as Epigallocatechin-3-gallate (EGCG), N-acetyl-L-cysteine (NAC) and N-tert-butyl-α-phenylnitrone (PBN)] reduce acute and chronic pruritus (14,15). We found that the pre-incubation of melatonin abrogated the increase of intracellular ROS in cultured ND7-23 cells induced by C48/80 or CQ. Thus, melatonin attenuated acute itch, perhaps by reducing the expressions of intracellular ROS in the neurons of DRG. Research has shown that CQ binds to MrgprA3 to activate TRPA1, leading to an increase of intracellular Ca2+ concentration in primary cultured DRG neurons (27). We also found that melatonin significantly inhibited the CQ-induced increase of intracellular Ca2+ concentration in the primary cultured DRG neurons. Similarly, melatonin has also been found to relieve pain by modulating Ca2+ influx in the DRG of diabetic rats (28).
Numerous studies have demonstrated that pro-inflammatory cytokines play important roles in chronic itch (3). For example, a specific receptor complex comprised of ST2 (also referred to as IL-1RL1) and the IL-1R accessory protein (IL-1RAP) binds to IL-33 and causes chronic pruritus (29,30). Finally, we found an increase of IL-33 and ST2 expression in the psoriasis and dry-skin mouse models. Melatonin inhibited the increase in lL-33/ST2 signaling in the mouse model of psoriasis. Conversely, the IL-31/IL-31ra pathway was increased in the dry-skin model, while melatonin was able to inhibit this increase.
Several methods of administration were used in acute itch model, including intraperitoneal (i.p), intradermal (i.d), and intrathecal (i.t). In order to detect the effect of systemic administration, we used an intraperitoneal (i.p) method of administration. To further elucidate the action site of melatonin, intradermal (i.d) and intrathecal (i.t). injection of melatonin were performed. It is possible that melatonin was acting at peripheral nerve terminals in intradermal (i.d) injection. The study showed that intrathecal (i.t) administration of melatonin has provided effective antipruritic effects, demonstrating that it may bind to melatonin receptors in the central nervous system.
This study had some limitations. The effects of melatonin were only examined in male mice; thus, female mice should be included in future studies. The mechanism of the effect also needs to be further investigated. In the present study, melatonin attenuates chronic itch in the mouse model of psoriasis and dry-skin model through the antioxidant and anti-inflammatory effects. However, the pathogenesis of chronic itch related to metabolic diseases remains elusive. But two pilot randomized, clinical trials demonstrated that patients with chronic liver disease and uremia had significant antipruritic effects when administered with melatonin daily (31,32).
Overall, we showed that melatonin treatment inhibited acute itch and chronic psoriatic and dry-skin pruritus in mice, possible by melatonin receptors, and its antioxidant, and anti-inflammatory properties. As demonstrated by our preclinical study, melatonin treatment may be an effective approach for treating itchy skin.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 81870874 and 82171229 to Tong Liu), the Nature Science Foundation of Jiangsu Province (No. BK20210839 to Guo-Kun Zhou), the Project of Jiangsu Provincial Health Commission (No. Z2020059 to Li Zhang), and the Medical Clinical Science and Technology Development Fund of Jiangsu University (No. JLY2021054 to Li Zhang).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3804/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-3804/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-3804/coif). LZ reports funding from the Project of Jiangsu Provincial Health Commission (No. Z2020059), and the Medical Clinical Science and Technology Development Fund of Jiangsu University (No. JLY2021054). GKZ reports funding from the Nature Science Foundation of Jiangsu Province (No. BK20210839). TL reports funding from the National Natural Science Foundation of China (Nos. 81870874 and 82171229). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Animal experiments were performed under a project license (No. 202101L0832) granted by institutional ethics board of Soochow University. All experiments were performed in strict accordance with Guidelines for Laboratory Animal Management issued by Soochow University.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ikoma A, Steinhoff M, Ständer S, et al. The neurobiology of itch. Nat Rev Neurosci 2006;7:535-47. [Crossref] [PubMed]
- Green D, Dong X. The cell biology of acute itch. J Cell Biol 2016;213:155-61. [Crossref] [PubMed]
- Dong X, Dong X. Peripheral and Central Mechanisms of Itch. Neuron 2018;98:482-94. [Crossref] [PubMed]
- Ji RR, Donnelly CR, Nedergaard M. Astrocytes in chronic pain and itch. Nat Rev Neurosci 2019;20:667-85. [Crossref] [PubMed]
- Roh YS, Choi J, Sutaria N, et al. Itch: Epidemiology, clinical presentation, and diagnostic workup. J Am Acad Dermatol 2022;86:1-14. [Crossref] [PubMed]
- Yosipovitch G, Bernhard JD. Clinical practice. Chronic pruritus. N Engl J Med 2013;368:1625-34. [Crossref] [PubMed]
- Foster RG. Melatonin. Curr Biol 2021;31:R1456-8. [Crossref] [PubMed]
- Masri S, Sassone-Corsi P. The emerging link between cancer, metabolism, and circadian rhythms. Nat Med 2018;24:1795-803. [Crossref] [PubMed]
- Zhang Y, Li X, Grailer JJ, et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res 2016;60:405-14. [Crossref] [PubMed]
- Hardeland R. Melatonin and inflammation-Story of a double-edged blade. J Pineal Res 2018;65:e12525. [Crossref] [PubMed]
- Taghavi Ardakani A, Farrehi M, Sharif MR, et al. The effects of melatonin administration on disease severity and sleep quality in children with atopic dermatitis: A randomized, double-blinded, placebo-controlled trial. Pediatr Allergy Immunol 2018;29:834-40. [Crossref] [PubMed]
- Kartha LB, Chandrashekar L, Rajappa M, et al. Serum melatonin levels in psoriasis and associated depressive symptoms. Clin Chem Lab Med 2014;52:e123-5. [Crossref] [PubMed]
- Chang YS, Lin MH, Lee JH, et al. Melatonin Supplementation for Children With Atopic Dermatitis and Sleep Disturbance: A Randomized Clinical Trial. JAMA Pediatr 2016;170:35-42. [Crossref] [PubMed]
- Zhou FM, Cheng RX, Wang S, et al. Antioxidants Attenuate Acute and Chronic Itch: Peripheral and Central Mechanisms of Oxidative Stress in Pruritus. Neurosci Bull 2017;33:423-35. [Crossref] [PubMed]
- Guo R, Zhou FM, Su CJ, et al. Epigallocatechin-3-gallate attenuates acute and chronic psoriatic itch in mice: Involvement of antioxidant, anti-inflammatory effects and suppression of ERK and Akt signaling pathways. Biochem Biophys Res Commun 2018;496:1062-8. [Crossref] [PubMed]
- Miyamoto T, Nojima H, Shinkado T, et al. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol 2002;88:285-92. [Crossref] [PubMed]
- Liu T, Xu ZZ, Park CK, et al. Toll-like receptor 7 mediates pruritus. Nat Neurosci 2010;13:1460-2. [Crossref] [PubMed]
- Imamachi N, Park GH, Lee H, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A 2009;106:11330-5. [Crossref] [PubMed]
- Akiyama T, Ivanov M, Nagamine M, et al. Involvement of TRPV4 in Serotonin-Evoked Scratching. J Invest Dermatol 2016;136:154-60. [Crossref] [PubMed]
- Chen Y, Fang Q, Wang Z, et al. Transient Receptor Potential Vanilloid 4 Ion Channel Functions as a Pruriceptor in Epidermal Keratinocytes to Evoke Histaminergic Itch. J Biol Chem 2016;291:10252-62. [Crossref] [PubMed]
- Wang XL, Tian B, Huang Y, et al. Hydrogen sulfide-induced itch requires activation of Cav3.2 T-type calcium channel in mice. Sci Rep 2015;5:16768. [Crossref] [PubMed]
- Than JY, Li L, Hasan R, et al. Excitation and modulation of TRPA1, TRPV1, and TRPM8 channel-expressing sensory neurons by the pruritogen chloroquine. J Biol Chem 2013;288:12818-27. [Crossref] [PubMed]
- Hill RZ, Loud MC, Dubin AE, et al. PIEZO1 transduces mechanical itch in mice. Nature 2022;607:104-10. [Crossref] [PubMed]
- Gu C, Wang F, Zhang YT, et al. Microglial MT1 activation inhibits LPS-induced neuroinflammation via regulation of metabolic reprogramming. Aging Cell 2021;20:e13375. [Crossref] [PubMed]
- Lin JJ, Lin Y, Zhao TZ, et al. Melatonin Suppresses Neuropathic Pain via MT2-Dependent and -Independent Pathways in Dorsal Root Ganglia Neurons of Mice. Theranostics 2017;7:2015-32. [Crossref] [PubMed]
- Cobb CA, Cole MP. Oxidative and nitrative stress in neurodegeneration. Neurobiol Dis 2015;84:4-21. [Crossref] [PubMed]
- Han L, Ma C, Liu Q, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 2013;16:174-82. [Crossref] [PubMed]
- Kahya MC, Nazıroğlu M, Övey İS. Modulation of Diabetes-Induced Oxidative Stress, Apoptosis, and Ca2+ Entry Through TRPM2 and TRPV1 Channels in Dorsal Root Ganglion and Hippocampus of Diabetic Rats by Melatonin and Selenium. Mol Neurobiol 2017;54:2345-60. [Crossref] [PubMed]
- Liu B, Tai Y, Achanta S, et al. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc Natl Acad Sci U S A 2016;113:E7572-9. [Crossref] [PubMed]
- Du L, Hu X, Yang W, et al. Spinal IL-33/ST2 signaling mediates chronic itch in mice through the astrocytic JAK2-STAT3 cascade. Glia 2019;67:1680-93. [Crossref] [PubMed]
- Baharvand P, Abbasi MR, Ziaee Ardestani S, et al. Evaluation of Antipruritic Effect of Melatonin on Hemodialysis Patients with Uremic Pruritus, A Double-Blind, Randomized, Crossover Trial. Iran J Kidney Dis 2021;1:38-47. [PubMed]
- Esmaeili A, Nassiri Toosi M, Taher M, et al. A Pilot Randomized, Clinical Trial of the Anti-pruritus Effect of Melatonin in Patients with Chronic Liver Disease. Iran J Pharm Res 2021;20:462-72. [PubMed]
(English Language Editor: L. Huleatt)


