Operative strategies for acute mitral regurgitation as a mechanical complication of myocardial infarction
Introduction
Acute mitral regurgitation (AMR) caused by papillary muscle rupture (PMR) represents a dramatic and life-threatening event after an acute myocardial infarction (AMI). With the advent of the percutaneous coronary intervention (PCI) reperfusion era, PMR has become increasingly rare, with an estimated incidence of 0.01–0.05% post-AMI (1,2). The clinical presentation varies from hemodynamic instability to cardiogenic shock and eventually cardiac arrest. A harsh and new consistent apical systolic murmur is the typical sign of acute valve dysfunction. Prompt diagnosis and management can lead to successful treatment for post-AMI PMR. Conservative treatment is usually futile leading to an in-hospital mortality as high as 80% (2). The poor results of such management make timely surgical intervention, though often challenging, mandatory (3). If rapid hemodynamic failure occurs, mechanical circulatory support (MCS), as a bridge to surgical or percutaneous treatment, plays a fundamental role, and the use of these devices is increasing in recent years (1). Despite the proposal of different surgical approaches, mitral valve replacement (MVR) is performed in the vast majority of patients, as compared to mitral valve repair (MVr) or trans-catheter mitral valve procedures, with almost half of the patients receiving concomitant coronary artery bypass grafting (CABG) (2). The aim of this review is to describe the approaches reported in the literature regarding the management of this rare disease.
History
In the first decades of 20th century, many books in cardiology did not mention post-AMI PMR. One of the first cases was reported at autopsy by John Hopkins University in 1935 (4). The literature first identifies ante-mortem PMR as early as 1948 (4). The terms and the description of “papillary muscle dysfunction” was introduced by Burch et al. (5). In 1964, Austen et al. (6) from Massachusetts General Hospital performed the first successful MVR for correction of PMR, and 2 years later, Horlick et al. (7). reported the first successful papillary muscle (PM) repair by means of reimplantation. Visualization of PMR via two-dimensional echocardiography was reported in 1981 and transesophageal echocardiography (TEE) was first used in 1985 in identifying the condition (8).
Pathogenesis
PMR is one of the most common causes of AMR. It presents within a week (1,2) after an AMI, and is associated with approximately 1% of deaths post-AMI (2). It develops, mostly, in patients who have had an inferior or posterior infarction. Indeed, the anterolateral PM has dual blood supply from the left anterior descending and left circumflex coronary arteries, whereas the posteromedial PM depends on single blood supply from the posterior descending artery bed. That is why posteromedial PMR is six to 12 times more frequent than anterolateral PMR (2,9). Rupture may be partial or complete, and survival largely depends on whether the mitral leaflets still have adequate blood supply. A complete disruption of the PM is seen in about one third of patients, resulting in acute flailing of both the anterior and the posterior mitral leaflets. Most reports describe worse outcome in patients developing complete PMR, probably because of its higher association with hemodynamic instability, pulmonary edema and more rapid development of cardiogenic shock (2). Occasionally, after an AMI, rupture of the chordae tendineae, is diagnosed. In this context, AMR is usually less pronounced than in the occasion of PMR, and surgical intervention could be, sometimes, less urgent, due to better a clinical condition at presentation (10). When chordae tendineae rupture occurs, the ischemic area is usually localized to the tip of the PM.
Operative technique
Preparations
The clinical presentation of post-AMI PMR can vary from dyspnea to signs of cardiogenic shock and, eventually, cardiac arrest. By auscultation, a harsh, consistent apical systolic murmur may be present (10). Although PMR is often fatal, the diagnosis is mandatory for timely surgery without further delay (3). Transthoracic echocardiography (TTE) is the “gold standard” and first line diagnostic method to identify PMR. It should be performed early in patients presenting with AMI and clinical suspicion of PMR to evaluate mitral valve abnormalities. Sensitivity with TTE, as diagnostic or suggestive of PMR, is around 90% (11). Identification of PMR and AMR may be demonstrated by echocardiography assessment. TTE may demonstrate a flail mitral valve leaflet with prolapse during systole into the atrium, visualization of a ruptured PM head with erratic movements in the ventricle or a mobile mass attached to the chordae tendineae. Four echo-criteria could be helpful in the diagnosis of PMR: (I) mobile density with erratic motion not correlating with ventricular motion within the left ventricle; (II) prolapse of mitral valve leaflets; (III) mitral regurgitation, and (IV) ventricular wall motion abnormalities. Reverse flow in the pulmonary vein is an insensitive, albeit specific indicator of severe AMR (11). TEE provides improved image quality of the mitral apparatus, when TTE is negative or not diagnostic. TEE provides clinically meaningful information to understand if eventual repair is suitable or not. A differential diagnosis must be made between AMR and the possibility of a ventricular septal defect (VSD) (10). The first-line management comprises of medical treatment, including administration of oxygen, restoration of fluid volume, correction of acidosis and the judicious administration of pressor amines and vasodilators, accompanied by monitoring of left-sided filling pressures and cardiac output (10). If the patient is still in hemodynamic instability or even cardiogenic shock, intra-aortic balloon pump (IABP) should be considered (12) and an upgrade to mechanical circulatory support might be taken into account. The European Society of Cardiology/European Association for Cardio-Thoracic Surgery Guidelines on myocardial revascularization allocate short-term mechanical circulatory support, in the presence of post-AMI mechanical complications, as a Class IIb recommendation (13). Extracorporeal Membrane Oxygenation (ECMO) may be useful to break the cycle, stabilize respiratory gas exchange and hemodynamic features and bridge patients safely to the operative room (14). The use of the Impella 2.5 (Abiomed, Danvers, Massechusetts), is also reported, to obtain direct unloading of the left ventricle (LV), increasing LV output and improving pulmonary edema (by decreasing retrograde mitral valve flow), but the risk of having the ruptured portion of the PM obstructing the impeller during suction should be taken into account (15). Coronary angiograms should be promptly performed, according to the clinical condition. Evaluation of the status of coronary disease would be helpful in decision making regarding eventual surgical or percutaneous (hybrid approach) revascularization, to obtain better outcomes and LV function (2,9).
Exposition
Once in the operating room, before chest skin incision, exposure of the femoral vessels and rapid preparation for the early institution of cardiopulmonary bypass (CBP) might be necessary to manage eventual hemodynamic instability, whenever prior mechanical circulatory support is not implanted. The most performed surgical access is a median sternotomy (with central or peripheral cannulation for CPB). As an alternative, if no other procedure besides the mitral approach will be performed and the patient’s condition permits, a mini thoracotomy at the IIIrd, IVth or Vth intercostal space (with peripheral CPB) could be considered. Myocardial protection is usually performed using anterograde or combined anterograde and retrograde cardioplegia. If a concomitant CABG is indicated, a distal anastomosis are performed (2,10). The mitral valve could be exposed with a left atriotomy or a trans-septal approach (16). In few case reports, when a combined procedure with LV restoration or LV ventriculotomy is necessary, a trans-ventricular mitral valve approach, avoiding an atriotomy and providing a larger operative mitral field, is used (17). After a MVR or MVr is executed and the exposition valve access is closed, the proximal anastomosis CABG can be completed.
Operation
Mitral valve replacement
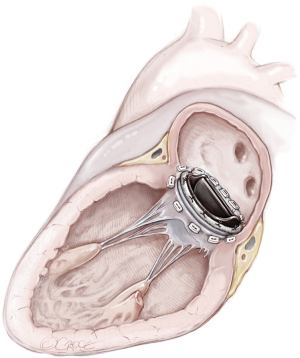
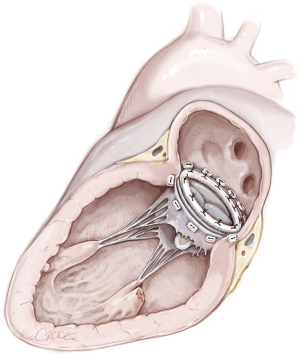
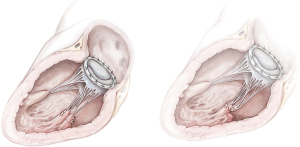
MVR, by mechanical or biological prosthesis implantation (Figures 1,2), still remains the most prevalent procedure in post-AMI PMR. In particular, it is usually performed for complete PMR and partial PMR associated with fragile necrotic tissue or in highly compromised patients to reduce surgical procedure times and their related risks (18). Low-profile prostheses are particularly recommended when infarcted tissue or aneurysmal resection is performed at the same time. Since the annular tissue may be weak, use of small Teflon® felt buttresses should be considered for support of the mattress sutures in order to prevent dehiscence and paravalvular leakage (10). Preservation of the subvalvular apparatus (“chordal sparing”) has become a standard procedure in MVR (19). It guarantees the continuity between the LV wall and the fibrous skeleton of the heart and maintains the ellipsoid geometry and function (19). A complete “chordal sparing” of one or both leaflets, which preserves the whole leaflet and associated first- and second-order chordae, has been described. As an alternative, partial “chordal sparing” provides an excision of a portion of the leaflet, preserving only third-order chordae (fixed to the annulus) (20). In PMR patients, a rare risk related to leaflet preservation is “sequential” PMR due to the traction and tearing at the remainder PM. Massive emboli could be generated from damaged muscle. In this case, a “double surgical approach” is advocated; the first type is a classic redo procedure with atrial access, prosthesis removal, resection of the second PMR and, in the end, a new valve replacement. Alternatively, to avoid the removal of the valve prosthesis another approach was described. The PMR is localized by an aortotomy and the help of an endoscopic 5 mm camera, the suture is passed through the fibrous part of the ruptured PM to improve its exposition. The chordae are then cut to resolve and remove it (20).
Mitral valve repair
MVr in the setting of PMR was introduced for preservation of LV function and improving LV remodeling and outcome (16,21). MVr could be considered in selected patients and several different techniques can be applied (16,21). The feasibility and the chosen method for surgical valve repair is usually dictated by the type of PMR, its surrounding tissues’ viability and the function of the LV wall at the base of PM (21). Several techniques have been described as follows.
Posterior mitral leaflet plication with or without combined posterior commissural annular plication
This approach was first reported by Adicoff et al. in 1963 (22) in partial PMR. The degree of mitral regurgitation and its incompetent area was reduced by a plication on itself with interrupted sutures, maintaining an intact chordae into apposition and removing the distal part with the residual PM detached. Additional sutures were placed in the mitral annulus to narrow the posterior commissure. Nowadays, this technique has been abandoned in favor of others that allow for better leaflet coaptation and greater stability of the annulus.
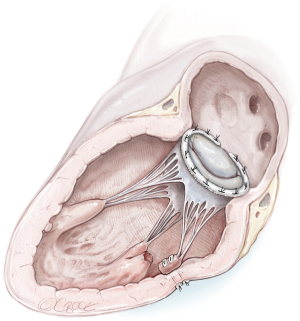
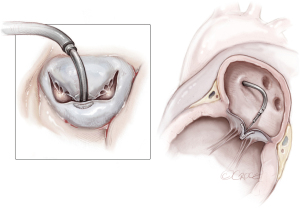
Reimplantation of the PM into the corresponding PM site and annuloplasty ring (Figure 3)
In the case of partial PMR, when mitral valve prolapse involves a segment that is too large to resect, the reimplantation technique of the remnant PM has been described (7,16,21,23). The reimplantation technique was introduced in 1966 and was successfully performed in partial PMR (7). The goal was to restore the fibrotic head of the PM in its own anatomical site with a single stitch suture. Previous identification of a resistant fibrous area is mandatory for a successful procedure. By the time, associated posterior mitral leaflet and annular plication evolved into posterior commissural annuloplasty and, in the end, a ring annuloplasty (23). Several precautions were described to strengthen the suture and avoid tissue damage, including the use of pledgeted sutures or pledget-supported horizontal mattress sutures (23,24). Reimplantation carries a higher risk of recurrence (23,25), as the success of reimplantation is critically dependent on the quality of the adjacent tissue (i.e., PM or LV) (7,16,25). Therefore, reimplantation of the ruptured head of the PM directly at the site of rupture is not advised. Retraction and involution of the edges of the reimplanted remnants can result in a shorter PM that experiences superior traction forces, rendering it prone to dehiscence, as shown by Jouan et al. (16,25).
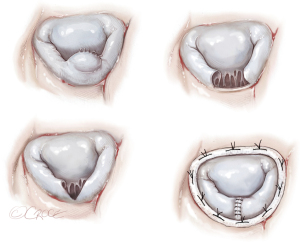
Reimplantation of the PM to the LV wall (Figure 4)
This type of repair should be considered in the presence of suitable tissue of the LV wall around the ruptured PM. A series of mattress sutures reinforced with Teflon felt could be inserted through the fibrous head of the PM and LV wall in such a manner to maintain the normal arrangement and tension of the chordae (23).
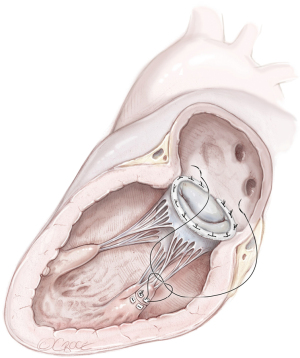
Reimplantation of the PM in the corresponding “healthy” PM and annuloplasty ring (Figure 5)
Height- and length-adjusted reimplantation of the PM tip in the corresponding viable PM is the preferred choice for reimplantation (21). It is the alternative to avoid risks related to PM reimplantation in the original PM site (16). Fasol et al. reported a series (six patients) of partial PMR treated with this technique. The MVr was described as a “sandwich pericardium-pledget-reinforced polytetrafluorethylene (PTFE, Gore-Tex sutures, WL Gore & Associates, Flagstaff, Arizona) sutures” to reimplant the PMR “height- and/or length-adjusted” into the corresponding PM. All procedures were associated by annuloplasty with a Carpentier-Edwards Physio-Annuloplasty Ring (Baxter Healthcare Corporation, Santa Ana, California) (21). Use of the Carpentier-Edwards Classic Ring was also reported (16). All patients had 0 or 1+ residual mitral regurgitation after a mean follow up of almost nine months (21).
Quadrangular or triangular resection of P2 and annuloplasty ring (Figure 6)
A segmental prolapse secondary to a partial PMR or ruptured chordae tendineae with limited adjacent tissue damage is often amenable to reliable repair techniques, such as quadrangular or triangular resection of a prolapsing mitral valve leaflet segment combined with annuloplasty (16,25). The Carpentier-Edwards Classic or Carbomedics ring were described in associated annuloplasty.
Chordal transfer and annuloplasty ring
Chordal transfer from the anterior to the posterior mitral leaflet in partial PMR was described once in the literature. The result was an intraoperative failure of the repair technique and resulted in a valve replacement (25).
Replacement of corresponding chordae with PTFE sutures and annuloplasty ring
When the PMR is located at the tip of the PM, expanded PTFE sutures can be used for replacement of diseased chordae tendineae during reconstructive procedures on the mitral valve, following the concept of “respect rather than resect” (26). Replacement of the primary chordae tendineae of the leaflet can be performed with either 4-0 or 5-0 polytetrafluoroethylene sutures. A double-armed suture is passed twice through the fibrous portion of the PM head and tied down. Each arm of the suture is then brought up to the free margin of the leaflet and passed through the area where the native chorda was attached. After the lengths of the two arms are adjusted, the ends are tied together (27). An alternative to the traditional surgical option is neochordae implantation; one of its advantages is their length adjustment through the beating heart (28) until a desired leaflet coaptation is reached. It was described by Sponga et al. (28) (Figure 7) in a posterior PMR. After placement of a double-armed PTFE mattress suture on the free border of the leaflet, non-infarcted tissue of the ventricular wall around the PMR was selected. The neochorda was passed through the ventricular wall from the endocardial to epicardial surface with a pledget and temporarily secured with a tourniquet. Then, the annuloplasty ring was implanted. The length of neochordae can be adjusted after declamping the aorta from the outside of the ventricle on the beating heart under the TEE guidance (28).
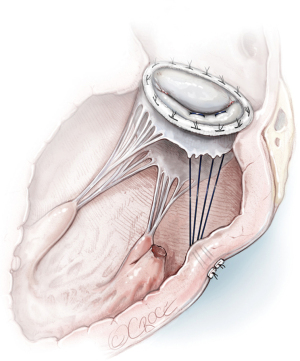
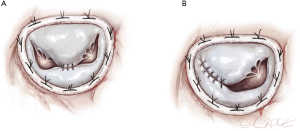
Surgical “Edge to Edge” repair (Figure 8A,8B)
Few data are reported concerning the “edge to edge” technique in PMR. The edge-to-edge technique was introduced as a method of MVr in the 1990s and has progressively been used to restore mitral competence in the setting of degenerative, post-endocarditis and functional mitral regurgitation. The free edges of the mitral leaflets must be approximated in correspondence to the site of the regurgitant jet in such a way that mitral regurgitation is corrected without producing stenosis. The rationale for its use could be considered in correlation with partial PMR or ruptures of chordae after an AMI or as a “rescue” in patients with significant residual mitral regurgitation after conventional mitral repair. A ring annuloplasty is always added to complete and stabilize the repair (29).
Percutaneous trans-catheter technique
High-risk surgical patients or inoperable subjects suffering from post-AMI AMR due to PMR still remain a major unsolved issue in the field of cardiovascular medicine and surgery. Percutaneous trans-catheter intervention (Figure 9), and in particular, the use of Mitraclip (Abbott Vascular, Santa Clara, CA, USA), has recently emerged and increased in temporal trend (30). Indeed, experienced centers introduced it as bail-out and “off label” strategy in high-risk surgical candidates in post-AMI AMR. The Mitraclip device has been extensively applied in degenerative etiology and it was considered recently for high-risk and inoperable patients. Despite encouraging data showing that Mitraclip may be successful in reducing the severity of AMR and reverse refractory cardiogenic shock in selected patients, this an alternative treatment in post-AMI PMR remains highly controversial and is most likely confined to a patient niche. Indeed, complications during the percutaneous approach, like a complete PMR with leaflet detachment from the necrotic ventricular wall, cannot be excluded. Therefore, concerning its safety and efficacy, more data and studies are warranted to conclusively indicate the actual potential and role of such an approach in post-AMI PMR (30).
The future?
In the hypothetical future prospects, in parallel with the increase use of the Mitraclip in post-AMI PMR, introduction of percutaneous valve replacement procedures is conceivable. It may represent an additional option in surgical high-risk patients who are also unsuitable for a trans-catheter repair. It is imaginable that this procedure could require ad hoc modifications in this field. A striking example is given by the need to remove the detached portions of the PM to reduce the risk of embolization of material before the valve positioning. Its hypothetical use remains fascinating, especially given the further potential therapeutic strategy in complex patients.
Comments
Different techniques have been developed over the years for the management of post-AMI AMR due to PMR. Although valve replacement is certainly the quickest and least complex choice, other surgical strategies have been described using valve/subvalvular reconstruction, in an attempt to prevent risks related to the prosthesis and to increase the possibility of a better outcome and a more physiologic cardiac function. However, no conclusive or consistent data regarding MVr technique for PMR has been shown to be safe and reproducible in the long term. Trans-catheter percutaneous procedures have been recently proposed in selected cases, like high-risk or inoperable patients, but further consideration for safety, indications and technical features, as well as efficacy in this peculiar setting should be addressed by future studies.
Acknowledgments
The authors thank Massimiliano (Max) Crespi for his help with developing the illustrations.
Funding: None.
Footnote
Conflicts of Interest: RL is a consultant for Medtronic, Getinge and LivaNova, and Member of the advisory board of Eurosets and Fresenius/Xenios. The other authors have no conflicts of interest to declare.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Elbadawi A, Elgendy IY, Mahmoud K, et al. Temporal Trends and Outcomes of Mechanical Complications in Patients With Acute Myocardial Infarction. JACC Cardiovasc Interv 2019;12:1825-36. [Crossref] [PubMed]
- Massimi G, Ronco D, De Bonis M, et al. Surgical treatment for post-infarction papillary muscle rupture: a multicentre study. Eur J Cardiothorac Surg 2022;61:469-76. [Crossref] [PubMed]
- Thompson CR, Buller CE, Sleeper LA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the SHOCK Trial Registry. SHould we use emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol 2000;36:1104-9. [Crossref] [PubMed]
- Moragues V. Spontaneous rupture of a papillary muscle of the heart. Report of case. Am Heart J 1939;17:106-10. [Crossref]
- Burch GE, De Pasquale NP, Phillips JH. Clinical manifestations of papillary muscle dysfunction. Arch Intern Med 1963;112:112-7. [Crossref] [PubMed]
- Austen WG, Sanders CA, Averill JH, et al. Ruptured papillary muscle. Report of a case with successful mitral valve replacement. Circulation 1965;32:597-601. [Crossref] [PubMed]
- Horlick L, Merriman JE, Robinson LN. A case of mitral insufficiency following myocardial infarction with rupture of a papillary muscle: improvement following reattachment of the papillary muscle and plication of the mitral valve. Can Med Assoc J 1966;94:192-5. [PubMed]
- Silveira I, Oliveira M, Gomes C, et al. Partial Papillary Muscle Rupture after Myocardial Infarction and Early Severe Obstructive Bioprosthetic Valve Thrombosis: an Unusual Combination. Arq Bras Cardiol 2018;111:430-3. [Crossref] [PubMed]
- Sharma SK, Seckler J, Israel DH, et al. Clinical, angiographic and anatomic findings in acute severe ischemic mitral regurgitation. Am J Cardiol 1992;70:277-80. [Crossref] [PubMed]
- Austen WG, McEnany MT. The role of surgery in the treatment of patients with complications of acute myocardial infarction. World J Surg 1978;2:709-16. [Crossref] [PubMed]
- Kerut EK, Hanawalt C, Everson C. Echo features of posteromedial papillary muscle rupture without papillary muscle prolapse into the left atrium. Echocardiography 2011;28:1046-8. [Crossref] [PubMed]
- Matteucci M, Fina D, Jiritano F, et al. Treatment strategies for post-infarction left ventricular free-wall rupture. Eur Heart J Acute Cardiovasc Care 2019;8:379-87. [Crossref] [PubMed]
- Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87-165. Erratum in: Eur Heart J 2019;40:3096. [Crossref] [PubMed]
- Staudacher DL, Bode C, Wengenmayer T. Severe mitral regurgitation requiring ECMO therapy treated by interventional valve reconstruction using the MitraClip. Catheter Cardiovasc Interv 2015;85:170-5. [Crossref] [PubMed]
- Jalil B, El-Kersh K, Frizzell J, et al. Impella percutaneous left ventricular assist device for severe acute ischaemic mitral regurgitation as a bridge to surgery. BMJ Case Rep 2017;2017:bcr-2017-219749. [Crossref] [PubMed]
- Bouma W, Wijdh-den Hamer IJ, Klinkenberg TJ, et al. Mitral valve repair for post-myocardial infarction papillary muscle rupture. Eur J Cardiothorac Surg 2013;44:1063-9. [Crossref] [PubMed]
- Cagli K, Gedik HS, Korkmaz K, et al. Transventricular mitral valve repair in patients with acute forms of ischemic mitral regurgitation. Tex Heart Inst J 2014;41:312-5. [Crossref] [PubMed]
- Lorusso R, Gelsomino S, De Cicco G, et al. Mitral valve surgery in emergency for severe acute regurgitation: analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg 2008;33:573-82. [Crossref] [PubMed]
- Ozdemir AC, Emrecan B, Baltalarli A. Bileaflet versus posterior-leaflet-only preservation in mitral valve replacement. Tex Heart Inst J 2014;41:165-9. [Crossref] [PubMed]
- de Cannière D, Vandenbossche JL, Nouar E, et al. Clinical Implications of Preserving Subvalvular Apparatus During Mitral Valve Replacement for Acute Ischemic Papillary Muscle Rupture. Ann Thorac Surg 2016;102:305-8. [Crossref] [PubMed]
- Fasol R, Lakew F, Wetter S. Mitral repair in patients with a ruptured papillary muscle. Am Heart J 2000;139:549-54. [Crossref] [PubMed]
- Adicoff A, Alexander CS, Ferguson JN, et al. Surgical repair of ruptured papillary muscle complicating posterior myocardial infarction. Am J Cardiol 1963;11:246-52. [Crossref] [PubMed]
- Rankin JS, Feneley MP, Hickey MS, et al. A clinical comparison of mitral valve repair versus valve replacement in ischemic mitral regurgitation. J Thorac Cardiovasc Surg 1988;95:165-77. [Crossref] [PubMed]
- Tahalele P, Prasmono A. Surgical repair of an impending rupture of left ventricular (LV) aneurysm with septal perforation and rupture of papillary muscle after acute myocarial infarction. Ann Thorac Cardiovasc Surg 2000;6:401-4. [PubMed]
- Jouan J, Tapia M, C, Cook R, et al. Ischemic mitral valve prolapse: mechanisms and implications for valve repair. Eur J Cardiothorac Surg 2004;26:1112-7. [Crossref] [PubMed]
- David TE. Techniques and results of mitral valve repair for ischemic mitral regurgitation. J Card Surg 1994;9:274-7. [Crossref] [PubMed]
- David TE, Bos J, Rakowski H. Mitral valve repair by replacement of chordae tendineae with polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg 1991;101:495-501. [Crossref] [PubMed]
- Sponga S, Tartara P, Vitali E, et al. Mitral valve repair after papillary muscle rupture through beating heart adjustment of artificial chordae length. Ann Thorac Surg 2010;90:e32-3. [Crossref] [PubMed]
- De Bonis M, Alfieri O. The edge-to-edge technique for mitral valve repair. HSR Proc Intensive Care Cardiovasc Anesth 2010;2:7-17. [PubMed]
- Papadopoulos K, Chrissoheris M, Nikolaou I, et al. Edge-to-edge mitral valve repair for acute mitral valve regurgitation due to papillary muscle rupture: a case report. Eur Heart J Case Rep 2019;3:ytz001. [Crossref] [PubMed]