Radial artery occlusion after transradial coronary catheterization
Introduction
Following the introduction of transradial coronary angiography by Campeau et al. in 1989 (1), Kiemeneij et al. were the first to document coronary angioplasty and stenting via the transradial approach (TRA) in 1993 (2). While the transfemoral approach (TFA) remains the most common method for coronary angiography and interventions, an increasing number of interventional cardiologists are performing percutaneous interventions through the radial artery (3-6). Furthermore, multiple studies have demonstrated significant benefit with TRA, due to its relatively lower potential for access site bleeding and high patient comfort/satisfaction, while maintaining an overall high procedural success rate (7-15). However, the increasing operators experience on TRA is followed by decreased experience in TFA, leading to more access site complications when this access site is chosen, the so called “Campeau Radial Paradox” (16).
Radial artery occlusion (RAO)
In the majority of cases performed through TRA, access site complications are predictable and easy to treat (17). New complications associated with TRA, like forearm pain or upper extremity loss of strength are under further evaluation in order to evaluate their impact on patients function and quality of life (18). However, treatment of complications after TRA depends on the experience of the interventional cardiologist performing the procedure. Potential access site complications during percutaneous procedures performed with a TRA are summarized in Table 1.
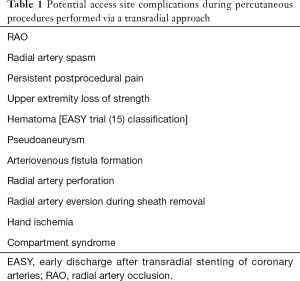
Full table
Pathogenesis of RAO
The most common complication of TRA is RAO, which occurs in about 1–10% of cases (19-22). Endothelial injury of the radial artery and decrease in blood flow after sheath and catheter insertion appear to contribute to thrombus formation and are predisposing factors for RAO (18,19,23). In addition, repeated radial artery cannulation can promote intimal hyperplasia and increased intima-media thickness (22,24,25), resulting in negative remodeling of the arterial wall and further predisposition to RAO (26). Radial artery stenosis has been shown to occur in 31% of patients within 2 days after TRA and in 28% late after the procedure (23). Imaging studies, such as vascular ultrasound (27), angiography (28), optical coherence tomography (24) and histopathological examination of materials aspirated after mechanical recanalization of occluded radial arteries (29) support this thrombus formation theory.
In most cases RAO occurs promptly after the procedure and up to 50% of patients have spontaneous recanalization of the artery within 1–3 months (22,30). Stella et al. found a 5.3% rate of RAO at the time of hospital discharge in a study of 563 patients who underwent transradial artery coronary angioplasty (20). RAO is, in the majority of patients, asymptomatic. This is due to dual blood supply of the hand and the usually rich network of collateral circulation: the radial and ulnar arteries undergo multiple anastomoses before they are connected in the hand by the superficial and deep palmar arches. Thus, if the radial artery is occluded, blood supply of the hand can be maintained by the ulnar collateral circulation and RAO is a quiescent event. However, cases of hand ischemia after RAO have been described in the setting of inadequate collateral circulation (31-33). Some patients may experience pain at the site of the occlusion, paresthesias or reduced limb function (23).
Eligibility for TRA
Traditionally, assessment of dual hand circulation to assess eligibility for transradial access is performed by the modified Allen’s test: while occlusive pressure to both the ulnar and the radial arteries is applied by the examiner’s fingers, the patient clenches their first for about one minute; the hand is then relaxed and pressure on the ulnar artery is released. Positive modified Allen’s test is recorded when the color of the hand is regained within 5–10 seconds, indicating adequate collateral circulation. These patients were traditionally considered as good candidates for TRA. However, new data makes us reconsider the use of this practice. We recently showed that TRA for coronary angiography and ad hoc angioplasty can be performed with similar efficacy and safety regardless of the preprocedural Allen’s test result (34). Barbeau’s test (35) is an alternative method for evaluating collateral circulation of the hand and is more objective compared with modified Allen’s test: a pulse oximeter is placed on the ipsilateral thumb and the morphology of the plethysmography tracing is examined after occlusion of the radial artery. Changes of the tracing are observed and categorized into four types. In type 4 there is absence of plethysmographic waveform indicating inadequate collateral circulation. However, neither modified Allen’s test, nor Barbeau’s test have been shown to predict clinically significant complications after TRA, making their value in clinical practice controversial (36). The most reliable method for assessing the collateral circulation before obtaining angiographic access is duplex ultrasonography (19). With this method the examiner can evaluate the blood flow in the arteries and collaterals and better understand their anatomy.
Diagnosis of RAO
Absence of radial pulse after TRA procedures may be a strong indicator of RAO. However, a palpable pulse does not exclude the diagnosis of RAO: collateral blood flow, through mainly the anterior interosseous artery, may supply the periphery of the radial artery distally to the occlusion, giving a false impression of radial artery patency. Therefore, RAO can often go undiagnosed, and its true prevalence may be underestimated. Radial artery patency is better evaluated with clinical testing as the reverse Barbeau’s test and with Doppler ultrasonography. In the former method, the ulnar artery is occluded and a pulse oximeter is placed on the ipsilateral thumb. Absence of plethysmographic waveforms is indicative of RAO. The latter method provides structural imaging of the arteries and assessment of blood flow with color Doppler ultrasound. Absence of flow in the radial artery suggests occlusion. The laser Doppler scan is a novel noninvasive method that may allow quick diagnosis of RAO (37).
Why is it important to maintain radial artery patency?
Many authors emphasize the importance of maintaining radial artery patency after TRA (18,19,38). A patent radial artery may be re-cannulated for future coronary artery procedures, used as conduit for coronary artery bypass grafting, arteriovenous fistula formation for hemodialysis in patients with end stage renal disease, or for intra-arterial pressure monitoring. Prior RAO has also been regarded as a contraindication for ipsilateral transulnar approach. However, Kedev et al. recently showed that transulnar approach can be safely performed by experienced operators even in patients with prior ipsilateral RAO (39). Agostoni et al. have also reported 6 cases of simultaneous radial and ipsilateral ulnar artery cannulation without any complications (40). A recently published consensus on TRA underlines the need of radial artery patency evaluation before discharge, as well as during the initial post-procedure follow-up visit (41).
Factors predisposing to RAO
Patient’s baseline characteristics and RAO
Many studies have evaluated baseline patient characteristics as predictors for RAO. Sex, age, diabetes, statin use, body weight, serum creatinine, and smoking have been evaluated; however, results are not consistent across all studies. Table 2 shows how these factors are associated with the incidence of RAO in present literature.
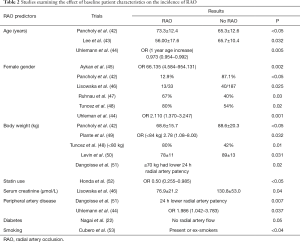
Full table
Procedural characteristics
In addition to clinical characteristics, procedural factors can predict and influence RAO incidence. Sheath size and its relation to radial artery diameter, as well as the utilization of specific pharmacological agents (such as anticoagulants and vasodilators) have been studied.
Sheath size and its relation to radial artery diameter
Bigger sheath sizes can lead to vascular damage and create a pro-thrombotic environment. Yoo et al. (54) found that the mean radial artery inner diameter was 2.69±0.40 mm in men and 2.43±0.38 mm in women. Given that the outer diameter of a 6F sheath is 2.52 mm, 32% of men and 60% of women in that study had radial artery diameter smaller than the outer diameter of a 6F sheath. In a Japanese study (30) the proportion of serious blood flow reduction in the radial artery was 13% when the outer sheath diameter was bigger than the inner artery diameter and 4% when the sheath diameter was smaller (P=0.01). Uhlemann et al. (44) found that the incidence of RAO was 13.7% in patients managed with 5F system compared with 30.5% in the group of 6F system (P<0.001). Other studies (55) have shown similar conclusions, underlining the importance of preserving a sheath-to-artery diameter ratio <1. In the Novel Angioplasty Using Coronary Accessor Trial (NAUSICA) patients were randomly assigned in a 1:1 ratio to undergo either 4F [using the KIWAMI, Heartrail II guide (Terumo, Tokyo, Japan), with an outer diameter of 1.43 mm] or 6F system (56). The primary endpoint was RAO at the next day of the procedure, defined as the absence of radial pulse confirmed by a negative reverse Allen’s test. Although the RAO rate was lower in the 4F versus the 6F groups, the difference was not statistically significant (0% vs. 4%, P=0.08). However, overall access site-related complications were significant lower in the 4F group (0% vs. 6%, P=0.02). Polimeni et al. (57) have recently published a meta-analysis of 11 studies (3 randomized and 8 nonrandomized) comparing the clinical and procedural outcomes of 5F versus 6F sheaths in transradial coronary interventions. They found no statistically significant difference in RAO incidence between the two groups [OR =0.88 (0.50–1.56), P=0.67]. Interestingly, in the meta-regression analysis on the influence of female sex on RAO, there was an increasing benefit with 5F sheath as the percentage of women included into the study increased (P=0.02).
Material miniaturization to prevent RAO
Despite the fact that the radial artery wall has elastic properties and can be stretched (19), the outer diameter of the sheath should be, whenever possible, smaller than the radial artery diameter during transradial procedures. Maintaining a sheath-to-artery ratio <1 is an essential factor in preventing RAO. Therefore, small size sheaths should be used for diagnostic angiography and for many non-complex coronary interventions, especially in women who have lower mean radial artery diameter compared to men. Hydrophilic sheaths may also reduce the risk of RAO (41). Sheathless guide catheters can reduce the outer diameter of vascular access system by 1−2 Fr compared with conventional sheaths and catheters (58,59). Yoshimachi et al. (60) studied the safety and feasibility of the new 5F Glidesheath Slender (Terumo), a hydrophilic coated introducer sheath, which has an inner diameter compatible with a conventional 5F guiding catheter, while the outer diameter is similar to that of a conventional 4F sheath. None of the 21 patients in the study experienced RAO.
Anticoagulation
In line with the thrombus formation theory of RAO, anticoagulant agents have been investigated as a means to reduce its incidence. Spaulding et al. (61) in a non-randomized study diagnosed RAO in 71% of patients who did not receive heparin, 24% of patients who received 2000−3,000 IU of heparin and 4.3% of patients who received 5,000 IU of heparin (P<0.05). Bernat et al. (62) studied the incidence of RAO in patients treated with either 2,000 or 5,000 IU of unfractionated heparin. Lower dose of heparin was associated with numerically double rates of RAO (5.9% vs. 2.9%, P=0.17). In the same study, when patients with RAO were treated with compression of the ipsilateral ulnar artery for 60 minutes, the incidence of RAO was reduced from 5.9% to 4.1% in the low-dose heparin group and from 2.9% to 0.8% in the high-dose heparin group (P=0.03). It has been suggested that heparin can be delivered either intravenously or via the arterial sheath having the same efficacy on reducing the incidence of RAO (63). Other anticoagulant agents that have been studied are enoxaparin (64) and bivalirudin (49,65). Plante et al. (49) compared bivalirudin versus heparin on RAO after transradial catheterization. In patients requiring angioplasty they administered a bolus 0.75 mg/kg of bivalirudin, followed by an infusion at a rate of 1.75 mg/kg/h. Patients who underwent only coronary angiography without angioplasty were given a bolus of 70 IU/kg of unfractionated heparin instead. They found no significant difference on the occurrence of RAO 4−8 weeks after the procedure (3.5% bivalirudin vs. 7.0% heparin, P=0.18). Hahalis et al. (66) randomized 308 consecutive patients undergoing transradial coronary angiography with 5F catheters to receive 2,500 or 5,000 IU of unfractionated heparin. The frequency of RAO between the two groups was similar (15.9% with low heparin dose vs. 14% with standard heparin dose, P=0.7). A case-control study by Pancholy et al. (67) compared patients receiving chronic oral anticoagulation with warfarin who underwent transradial coronary angiography without parenteral anticoagulation with non-warfarinized patients who received an intravenous heparin bolus (50 IU/kg). Patients under warfarin had a higher incidence of early (24 hours) and late (30 days) RAO compared with the heparin group (18.6% vs. 9.6%, P=0.024 and 13.9% vs. 5.2%, P=0.01, respectively). In a recently published systematic review and meta-analysis (68) it was found that the most significant measure that decreased RAO was higher doses of heparin (risk ratio 0.36, 95% CI: 0.17–0.76). The recommended dose of unfractionated heparin is at least 50 IU/kg, up to 5,000 IU (41). Bivalirudin should be administered at a dose of 0.75 mg/kg bolus intravenously for diagnostic procedures, followed by infusion at 1.75 mg/kg/h if PCI is indicated (41). Enoxaparin, at a 60 mg dose (41), can be also used via the arterial sheath.
Vasodilators and RAO
Many vasodilator agents have been used during TRA for prevention of radial artery spasm and RAO, including nitrates, calcium channel blockers, lidocaine, magnesium sulphate and alpha blockers. Increase of peri-procedural radial artery diameter was demonstrated in a series of studies (69-72). Dharma et al. (73) found that the use of post-procedural/pre-hemostasis intra-arterial nitroglycerin reduced the incidence of RAO compared with placebo (8.3% vs. 11.7%, P=0.006). A systematic review by Kwok et al. (74) analyzed the rate of radial artery spasm in 22 clinical studies. They concluded that 5 mg of verapamil or verapamil in combination with nitroglycerin are the most effective measures to prevent radial artery spasm. However, in a study by Izgi et al. (75), none of 15 consecutive patients who were treated with TRA without vasodilators experienced RAO. Further investigation is needed to elucidate the optimal use of vasodilator regimens for the prevention of RAO. Table 3 summarizes the effect of procedural characteristics on the incidence of RAO in current literature.
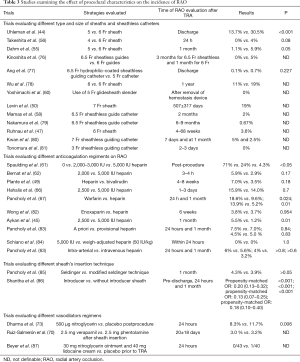
Full table
Post-procedural care
Patent hemostasis
The term “patent hemostasis” is used to describe patency of the radial artery while hemostasis at the site of puncture is achieved with a hemostatic device. This non-occlusive compression of the radial artery has been recognized as an independent predictor of radial artery patency after TRA. In the Prevention of Radial Artery Occlusion-Patent Hemostasis Evaluation Trial (PROPHET), 436 consecutive patients undergoing transradial catheterization were randomized between conventional hemostasis and patent hemostasis (42). Radial artery patency, assessed using the reverse Barbeau’s test, was evaluated at 24 hours and 1 month after the procedure. There was a significant reduction of 24 hours RAO (5% vs. 12%, P<0.05) and 1 month RAO (1.8% vs. 7%, P<0.05) in the patent hemostasis group compared with the conventional hemostasis group. The Radial Compression Guided by Mean Artery Pressure Versus standard Compression with a Pneumatic Device (RACOMAP) trial has also shown the importance of patent hemostasis (53). In this trial, patients who received compression guided by mean arterial pressure had significant reduction of RAO incidence compared with patients who received a standard compression with 15 cc of air in the bladder of the TR Band device (Terumo, Somerset, NJ, USA) (1.2% vs. 12%, P=0.0001). In a recently published study (88), patients managed with standard compression of the radial artery for 2 hours using a TR Band device (the air bladder of the device was filled initially with 18 mL of air, then deflated until pulsatile bleeding occurred and 2 mL of air re-introduced in the bladder to achieve hemostasis) were compared with patients who received the rapid deflation technique. In this technique, exactly 15 minutes after TR Band application, radial artery patency was evaluated using the reverse Barbeau’s test; the compression device was then deflated to the minimum volume of 7 mL while hemostasis was maintained. If bleeding occurred, 2 mL of air was re-introduced, radial artery patency was documented and the TR Band was removed after 2 hours. RAO was assessed 24 hours after the procedure, and was significantly lower in the rapid deflation technique group compared with the group receiving conventional hemostasis (2% vs. 14.9%, P=0.002).
Based on the above and other data, the Society for Cardiovascular Angiography and Intervention’s (SCAI) Transradial Working Group has suggested that patent hemostasis should be used in all patients who undergo transradial procedures (41). The following process is proposed: withdrawal of the arterial sheath 2–3 cm, application of the hemostatic compression device 2–3 mm proximal to the skin entry site and removal of the sheath after tightening the device. Afterwards, the operator should decrease the pressure of the compression device to the point of mild pulsatile bleeding at the skin entry site and after 2–3 cycles of pulsatile bleeding retighten the device gradually to eliminate this pulsatile bleeding. Finally, radial artery patency is evaluated by using the reverse Barbeau’s test.
Novel techniques that improve radial artery patency are under investigation. Simultaneous compression of the ipsilateral ulnar artery during radial artery patent hemostasis (the so called ULTRA technique), which increases blood flow velocity in the radial artery (89), while maintaining non-occlusive radial hemostasis showed promising results in a non-randomized study (90). Furthermore, in the recently published Prophylactic Hyperperfusion Evaluation Trial (PROPHET-II), ipsilateral ulnar artery compression, while applying patent hemostasis on the radial artery reduced the risk of RAO compared with the conventional patent hemostasis group at 24 hours (1.0% vs. 4.3%, P=0.0001) and at 30 days (0.9% vs. 3.0%, P=0.0001) (91).
Post-procedure compression duration
The duration of post-procedure compression has also been shown to be important: a study (92) has shown that only 2 hours of compression after the removal of the arterial sheath significantly reduced the risk of RAO 24 hours after the procedure as compared with the 6 hours of compression(5.5% vs. 12%, P=0.025). Another randomized study (73) demonstrated that >4 hours of compression increased the risk of RAO compared to <4 hours (OR 3.11, 95% CI: 1.66−5.82, P<0.001). Politi et al. (93) found that 15 minutes of post-procedure compression reduced the incidence of RAO compared with 2 hours (5% vs. 10%, P=0.05). Different hemostatic devices have been compared as well. Pancholy et al. (94) found that the use of the inflatable TR Band compression device reduced the risk of RAO at 24 hours (4.4% vs. 11.2%, P<0.005) and at 30 days (3.2% vs. 7.2%, P<0.05) after the procedure compared with the HemoBand device.
Treatment of RAO
While RAO is a usually subclinical condition and can be managed conservatively, in some cases active treatment may be needed. Zankl et al. (95) treated early symptomatic RAO with enoxaparin or fondaparinux for 4 weeks. After 1 month 87% of patients had a recanalized radial artery. Bernat et al. (62) compared RAO in patients undergoing TRA to receive 2,000 or 5000 IU of heparin. Occurrence of RAO was not statistically significant between the two groups (5.9% vs. 2.9%, P=0.17), but when treatment with 1-hour ipsilateral ulnar artery compression was applied, the risk of RAO was reduced in the 5,000 IU group (4.1% vs. 0.8%, P=0.03). Percutaneous techniques have also been used to treat RAO. Recanalization of the occluded radial artery with angioplasty has been described in a number of studies (28,29,32). The occluded part can be approached from the distal radial artery, the palmar arch or antegradely from the brachial artery.
Conclusions
TRA for coronary angiography and interventions has many benefits compared with the transfemoral approach. Its most important complication is RAO which could be a discouraging issue for many operators. Routine use of patent hemostasis, higher dose of anticoagulation and shorter post-procedure compression time have been shown to reduce the risk of RAO. All patients should be examined for radial artery patency before discharge. Novel studies and techniques are needed to improve strategies that minimize the incidence of this major complication of radial access.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to disclosure.
References
- Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn 1989;16:3-7. [Crossref] [PubMed]
- Kiemeneij F, Laarman GJ, de Melker E. Transradial artery coronary angioplasty. Am Heart J 1995;129:1-7. [Crossref] [PubMed]
- Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv 2008;1:379-86. [Crossref] [PubMed]
- Asrar Ul Haq M, Tsay IM, Dinh DT, et al. Prevalence and outcomes of trans-radial access for percutaneous coronary intervention in contemporary practise. Int J Cardiol 2016;221:264-8. [Crossref] [PubMed]
- Baklanov DV, Kaltenbach LA, Marso SP, et al. The prevalence and outcomes of transradial percutaneous coronary intervention for ST-segment elevation myocardial infarction: analysis from the National Cardiovascular Data Registry (2007 to 2011). J Am Coll Cardiol 2013;61:420-6. [Crossref] [PubMed]
- Gutierrez A, Tsai TT, Stanislawski MA, et al. Adoption of Transradial Percutaneous Coronary Intervention and Outcomes According to Center Radial Volume in the Veterans Affairs Healthcare System Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking (CART) Program. Circ Cardiovasc Interv 2013;6:336-46. [Crossref] [PubMed]
- Doyle BJ, Rihal CS, Gastineau DA, et al. Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: implications for contemporary practice. J Am Coll Cardiol 2009;53:2019-27. [Crossref] [PubMed]
- Jolly SS, Amlani S, Hamon M, et al. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J 2009;157:132-40. [Crossref] [PubMed]
- Agostoni P, Biondi-Zoccai GG, De Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol 2004;44:349-56. [Crossref] [PubMed]
- Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet 2011;377:1409-20. [Crossref] [PubMed]
- Romagnoli E, Biondi-Zoccai G, Sciahbasi A, et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol 2012;60:2481-9. [Crossref] [PubMed]
- Valgimigli M, Gagnor A, Calabró P, et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet 2015;385:2465-76. [Crossref] [PubMed]
- Komócsi A, Aradi D, Kehl D, et al. Meta-analysis of randomized trials on access site selection for percutaneous coronary intervention in ST-segment elevation myocardial infarction. Arch Med Sci 2014;10:203-12. [Crossref] [PubMed]
- Bertrand OF, De Larochelliere R, Rodes-Cabau J, et al. Early Discharge After Transradial Stenting of Coronary Arteries Study Investigators A randomized study comparing same-day home discharge and abciximab bolus only to overnight hospitalization and abciximab bolus and infusion after transradial coronary stent implantation. Circulation 2006;114:2636-43. [Crossref] [PubMed]
- Cooper CJ, El-Shiekh RA, Cohen DJ, et al. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J 1999;138:430-6. [Crossref] [PubMed]
- Azzalini L, Tosin K, Chabot-Blanchet M, Avram R, et al. The Benefits Conferred by Radial Access for Cardiac Catheterization Are Offset by a Paradoxical Increase in the Rate of Vascular Access Site Complications With Femoral Access: The Campeau Radial Paradox. JACC Cardiovasc Interv 2015;8:1854-64. [Crossref] [PubMed]
- Sławin J, Kubler P, Szczepański A, et al. Radial artery occlusion after percutaneous coronary interventions - an underestimated issue. Postepy Kardiol Interwencyjnej 2013;9:353-61. [Crossref] [PubMed]
- Zwaan EM, IJsselmuiden AJ, van Rosmalen J, et al. Rationale and design of the ARCUS: Effects of trAnsRadial perCUtaneouS coronary intervention on upper extremity function. Catheter Cardiovasc Interv 2016;88:1036-1043. [Crossref] [PubMed]
- Kotowycz MA, Džavík V. Radial artery patency after transradial catheterization. Circ Cardiovasc Interv 2012;5:127-33. [Crossref] [PubMed]
- Stella PR, Kiemeneij F, Laarman GJ, et al. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet Cardiovasc Diagn 1997;40:156-8. [Crossref] [PubMed]
- Zhou YJ, Zhao YX, Cao Z, et al. Incidence and risk factors of acute radial artery occlusion following transradial percutaneous coronary intervention. Zhonghua Yi Xue Za Zhi 2007;87:1531-4. [PubMed]
- Nagai S, Abe S, Sato T, et al. Ultrasonic assessment of vascular complications in coronary angiography and angioplasty after transradial approach. Am J Cardiol 1999;83:180-6. [Crossref] [PubMed]
- Wagener JF, Rao SV. Radial artery occlusion after transradial approach to cardiac catheterization. Curr Atheroscler Rep 2015;17:489. [Crossref] [PubMed]
- Yonetsu T, Kakuta T, Lee T, et al. Assessment of acute injuries and chronic intimal thickening of the radial artery after transradial coronary intervention by optical coherence tomography. Eur Heart J 2010;31:1608-15. [Crossref] [PubMed]
- Wakeyama T, Ogawa H, Iida H, et al. Intima-media thickening of the radial artery after transradial intervention: an intravascular ultrasound study. J Am Coll Cardiol 2003;41:1109-14. [Crossref] [PubMed]
- Sakai H, Ikeda S, Harada T, et al. Limitations of successive transradial approach in the same arm: the Japanese experience. Catheter Cardiovasc Interv 2001;54:204-8. [Crossref] [PubMed]
- Kim KS, Park HS, Jang WI, et al. Thrombotic occlusion of the radial artery as a complication of the transradial coronary intervention. J Cardiovasc Ultrasound 2010;18:31. [Crossref] [PubMed]
- Babunashvili A, Dundua D. Recanalization and reuse of early occluded radial artery within 6 days after previous transradial diagnostic procedure. Catheter Cardiovasc Interv 2011;77:530-6. [Crossref] [PubMed]
- Pancholy SB. Transradial access in an occluded radial artery: new technique. J Invasive Cardiol 2007;19:541-4. [PubMed]
- Saito S, Ikei H, Hosokawa G, et al. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv 1999;46:173-8. [Crossref] [PubMed]
- Rademakers LM, Laarman GJ. Critical hand ischaemia after transradial cardiac catheterisation: an uncommon complication of a common procedure. Neth Heart J 2012;20:372-5. [Crossref] [PubMed]
- Ruzsa Z, Kovács N, Merkely B. Retrograde subintimal recanalization of a radial artery occlusion after coronary angiography using the palmar loop technique. Cardiovasc Revasc Med 2015;16:259-61. [Crossref] [PubMed]
- Ayan M, Smer A, Azzouz M, et al. Hand ischemia after transradial coronary angiography: Resulting in right ring finger amputation. Cardiovasc Revasc Med 2015;16:367-9. [Crossref] [PubMed]
- Maniotis C, Koutouzis M, Andreou C, et al. Transradial Approach for Cardiac Catheterization in Patients With Negative Allen's Test. J Invasive Cardiol 2015;27:416-20. [PubMed]
- Barbeau GR, Arsenault F, Dugas L, et al. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen's test in 1010 patients. Am Heart J 2004;147:489-93. [Crossref] [PubMed]
- Bertrand OF, Rao SV, Pancholy S, et al. Transradial approach for coronary angiography and interventions: results of the first international transradial practice survey. JACC Cardiovasc Interv 2010;3:1022-31. [Crossref] [PubMed]
- De Rosa S, Passafaro F, Polimeni A, et al. A novel quick and easy test for radial artery occlusion with the laser Doppler scan. JACC Cardiovasc Interv 2014;7:e89-90. [Crossref] [PubMed]
- Amin H. Prevention of radial artery occlusion: it's the right thing to do. EuroIntervention 2015;11:731-3. [Crossref] [PubMed]
- Kedev S, Zafirovska B, Dharma S, et al. Safety and feasibility of transulnar catheterization when ipsilateral radial access is not available. Catheter Cardiovasc Interv 2014;83:E51-60. [Crossref] [PubMed]
- Agostoni P, Zuffi A, Faurie B, et al. Same wrist intervention via the cubital (ulnar) artery in case of radial puncture failure for percutaneous cardiac catheterization or intervention: the multicenter SWITCH registry. Int J Cardiol 2013;169:52-6. [Crossref] [PubMed]
- Rao SV, Tremmel JA, Gilchrist IC, et al. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and intervention's transradial working group. Catheter Cardiovasc Interv 2014;83:228-36. [Crossref] [PubMed]
- Pancholy S, Coppola J, Patel T, et al. Prevention of radial artery occlusion—patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Catheter Cardiovasc Interv 2008;72:335-40. [Crossref] [PubMed]
- Lee WC, Chen HC, Fang CY, et al. Incidence and predictors of radial artery occlusion after using sheathless standard guiding catheters in complex coronary intervention and carotid artery stenting by trans-radial approach. Exp Clin Cardiol 2014;20:1305-27.
- Uhlemann M, Möbius-Winkler S, Mende M, et al. The Leipzig prospective vascular ultrasound registry in radial artery catheterization: impact of sheath size on vascular complications. JACC Cardiovasc Interv 2012;5:36-43. [Crossref] [PubMed]
- Aykan AÇ, Gökdeniz T, Gül I, et al. Comparison of low dose versus standard dose heparin for radial approach in elective coronary angiography?. Int J Cardiol 2015;187:389-92. [Crossref] [PubMed]
- Lisowska A, Knapp M, Tycińska A, et al. Radial access during percutaneous interventions in patients with acute coronary syndromes: should we routinely monitor radial artery patency by ultrasonography promptly after the procedure and in long-term observation?. Int J Cardiovasc Imaging 2015;31:31-6. [Crossref] [PubMed]
- Ruhnau J, Schroder S. Prevalence of and risk factors for radial artery complications after transradial cardiac catheterization. Circulation. 2013;128:A18953.
- Tuncez A, Kaya Z, Aras D, et al. Incidence and predictors of radial artery occlusion associated transradial catheterization. Int J Med Sci 2013;10:1715-19. [Crossref] [PubMed]
- Plante S, Cantor WJ, Goldman L, et al. Comparison of bivalirudin versus heparin on radial artery occlusion after transradial catheterization. Catheter Cardiovasc Interv 2010;76:654-8. [Crossref] [PubMed]
- Levin D, Adawi S, Halon D, et al. Long--term radial artery patency following transradial coronary catheterisation via a 7F--sheath. IMAJ 2016;18:290-3. [PubMed]
- Dangoisse V, Guédès A, Chenu P, et al. Radial artery patency after transradial access: effective and easy way to reduce the radial artery occlusion rate, results of the CRASOC (Compression of Radial Arteries Without Occlusion) Study. J Am Coll Cardiol 2012;59:E193. [Crossref]
- Honda T, Fujimoto K, Miyao Y, et al. Access site-related complications after transradial catheterization can be reduced with smaller sheath size and statins. Cardiovasc Interv Ther 2012;27:174-80. [Crossref] [PubMed]
- Cubero JM, Lombardo J, Pedrosa C, et al. Radial compression guided by mean artery pressure versus standard compression with a pneumatic device (RACOMAP). Catheter Cardiovasc Interv 2009;73:467-72. [Crossref] [PubMed]
- Yoo BS, Yoon J, Ko JY, et al. Anatomical consideration of the radial artery for transradial coronary procedures: arterial diameter, branching anomaly and vessel tortuosity. Int J Cardiol 2005;101:421-7. [Crossref] [PubMed]
- Dahm JB, Vogelgesang D, Hummel A, et al. A randomized trial of 5 vs. 6 French transradial percutaneous coronary interventions. Catheter Cardiovasc Interv 2002;57:172-6. [Crossref] [PubMed]
- Takeshita S, Saito S, Hata T, et al. Comparison of Frequency of Radial Artery Occlusion after 4-Fr versus 6-Fr Transradial Coronary Intervention from the NAUSICA (Novel Angioplasty USIng Coronary Accessor) Trial. Am J Cardiol 2014;113:1986-9. [Crossref] [PubMed]
- Polimeni A, Passafaro F, De Rosa S, et al. Clinical and Procedural Outcomes of 5-French versus 6-French Sheaths in Transradial Coronary Interventions. Medicine 2015;94:e2170. [Crossref] [PubMed]
- Mamas M, D'Souza S, Hendry C, et al. Use of the sheathless guide catheter during routine transradial percutaneous coronary intervention: a feasibility study. Catheter Cardiovasc Interv 2010;75:596-602. [PubMed]
- Youn YJ, Yoon J, Han SW, et al. Feasibility of transradial coronary intervention using a sheathless guiding catheter in patients with small radial artery. Korean Circ J 2011;41:143-8. [Crossref] [PubMed]
- Yoshimachi F, Kiemeneij F, Masutani M, et al. Safety and feasibility of the new 5 Fr Glidesheath Slender. Cardiovasc Interv Ther 2016;31:38-41. [Crossref] [PubMed]
- Spaulding C, Lefevre T, Funck F, et al. Left radial approach for coronary angiography: results of a prospective study. Cathet Cardiovasc Diagn 1996;39:365-70. [Crossref] [PubMed]
- Bernat I, Bertrand OF, Rokyta R, et al. Efficacy and safety of transient ulnar artery compression to recanalize acute radial artery occlusion after transradial catheterization. Am J Cardiol 2011;107:1698-701. [Crossref] [PubMed]
- Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol 2009;104:1083-5. [Crossref] [PubMed]
- Feray H, Izgi C, Cetiner D, et al. Effectiveness of enoxaparin for prevention of radial artery occlusion after transradial cardiac catheterization. J Thromb Thrombolysis 2010;29:322-5. [Crossref] [PubMed]
- Venkatesh K, Mann T. Transitioning from heparin to bivalirudin in patients undergoing ad hoc transradial interventional procedures: a pilot study. J Invasive Cardiol 2006;18:120-4. [PubMed]
- Hahalis G, Xathopoulou I, Tsigkas G, et al. A comparison of low versus standard heparin dose for prevention of forearm artery occlusion after 5 French coronary angiography. Int J Cardiol 2015;187:404-10. [Crossref] [PubMed]
- Pancholy SB, Ahmed I, Bertrand OF, et al. Frequency of radial artery occlusion after transradial access in patients receiving warfarin therapy and undergoing coronary angiography. Am J Cardiol 2014;113:211-4. [Crossref] [PubMed]
- Rashid M, Kwok CS, Pancholy S, et al. Radial Artery Occlusion After Transradial Interventions: A Systematic Review and Meta-Analysis. J Am Heart Assoc 2016;5:e002686. [Crossref] [PubMed]
- Abe S, Meguro T, Endoh N, et al. Response of the radial artery to three vasodilatory agents. Catheter Cardiovasc Interv 2000;49:253-6. [Crossref] [PubMed]
- Ruiz-Salmerón RJ, Mora R, Masotti M, et al. Assessment of the efficacy of phentolamine to prevent radial artery spasm during cardiac catheterization procedures: a randomized study comparing phentolamine vs. verapamil. Catheter Cardiovasc Interv 2005;66:192-8. [Crossref] [PubMed]
- Kim SH, Kim EJ, Cheon WS, et al. Comparative study of nicorandil and a spasmolytic cocktail in preventing radial artery spasm during transradial coronary angiography. Int J Cardiol 2007;120:325-30. [PubMed]
- Byrne J, Spence M, Haegeli L, et al. Magnesium sulphate during transradial cardiac catheterization: a new use for an old drug? J Invasive Cardiol 2008;20:539-42. [PubMed]
- Dharma S, Kedev S, Patel T, et al. A novel approach to reduce radial artery occlusion after transradial catheterization: Postprocedural/prehemostasis intra-arterial nitroglycerin. Catheter Cardiovasc Interv 2015;85:818-25. [Crossref] [PubMed]
- Kwok CS, Rashid M, Fraser D, et al. Intra-arterial vasodilators to prevent radial artery spasm: a systematic review and pooled analysis of clinical studies. Cardiovasc Revasc Med 2015;16:484-90. [Crossref] [PubMed]
- Izgi C, Feray H. Is Radial Access and Transradial Cardiac Catheterization Feasible without the Use of Any Vasodilator? Int J Angiol 2014;23:41-6. [Crossref] [PubMed]
- Kinoshita N, Ota K, Yamada T, et al. AS-155 Useful of the Sheathless Guide Catheter during Routine Transradial Percutaneous Coronary Intervention. Am J Cardiol 2011;107:45A. [Crossref]
- Ang CY, Dinh HL, Lontoc AN, et al. TCT-299 Comparison of Slender (6.5 French Sheathless) Guiding Catheters Versus 5 French Guiding Catheters for Slender Transradial Coronary Intervention. J Am Coll Cardiol 2013;62:B96-7. [Crossref]
- Wu SS, Galani RJ, Bahro A, et al. 8 french transradial coronary interventions: clinical outcome and late effects on the radial artery and hand function. J Invasive Cardiol 2000;12:605-9. [PubMed]
- Nakamura S, Oshima S, Noda K, et al. Safety and efficacy of transradial coronary intervention using a 6.5 Fr sheathless guide catheter. Eur Heart J 2011;32:399.
- Kwan TW, Cherukuri S, Huang Y, et al. Feasibility and safety of 7F sheathless guiding catheter during transradial coronary intervention. Catheter Cardiovasc Interv 2012;80:274-80. [Crossref] [PubMed]
- Tonomura D, Shimada Y, Yano K, et al. Feasibility and safety of a virtual 3-Fr sheathless-guiding system for percutaneous coronary intervention. Catheter Cardiovasc Interv 2014;84:426-35. [Crossref] [PubMed]
- Wong CP, Ho HH, Tan JK, et al. AS-292: Radial Artery Patency Post Percutaneous Coronary Intervention with Intravenous Enoxaparin as Procedural Antocoagulant in an Asian Population. Am J Cardiol 2012;109:S142. [Crossref]
- Pancholy SB, Bertrand OF, Patel T. Comparison of a priori versus provisional heparin therapy on radial artery occlusion after transradial coronary angiography and patent hemostasis (from the PHARAOH Study). Am J Cardiol 2012;110:173-6. [Crossref] [PubMed]
- Schiano P, Barbou F, Chenilleau MC, et al. Adjusted weight anticoagulation for radial approach in elective coronarography: the AWARE coronarography study. EuroIntervention 2010;6:247-50. [Crossref] [PubMed]
- Pancholy SB, Sanghvi KA, Patel TM. Radial artery access technique evaluation trial: randomized comparison of Seldinger versus modified Seldinger technique for arterial access for transradial catheterization. Catheter Cardiovasc Interv 2012;80:288-91. [Crossref] [PubMed]
- Shantha GP, Pancholy SB. Comparison of incidence of radial artery occlusion in patients undergoing trans-radial intervention with or without a sheath: a prospective cohort study. Circulation 2014;130:A13877.
- Beyer AT, Ng R, Singh A, et al. Topical nitroglycerin and lidocaine to dilate the radial artery prior to transradial cardiac catheterization: a randomized, placebo-controlled, double-blind clinical trial: the PRE-DILATE Study. Int J Cardiol 2013;168:2575-8. [Crossref] [PubMed]
- Edris A, Gordin J, Sallam T, et al. Facilitated patent haemostasis after transradial catheterisation to reduce radial artery occlusion. EuroIntervention 2015;11:765-71. [Crossref] [PubMed]
- Tian J, Chu YS, Sun J, et al. Ulnar Artery Compression: A Feasible and Effective Approach to Prevent the Radial Artery Occlusion after Coronary Intervention. Chin Med J 2015;128:795. [Crossref] [PubMed]
- Koutouzis MJ, Maniotis CD, Avdikos G, et al. ULnar Artery Transient Compression Facilitating Radial Artery Patent Hemostasis (ULTRA): A Novel Technique to Reduce Radial Artery Occlusion After Transradial Coronary Catheterization. The J Invasive Cardiol 2016;28:451-4. [PubMed]
- Pancholy SB, Bernat I, Bertrand OF, et al. Prevention of radial artery occlusion after transradial catheterization: the PROPHET-II randomized trial. JACC Cardiovasc Interv 2016;9:1992-9. [Crossref] [PubMed]
- Pancholy SB, Patel TM. Effect of duration of hemostatic compression on radial artery occlusion after transradial access. Catheter Cardiovasc Interv 2012;79:78-81. [Crossref] [PubMed]
- Politi L, Aprile A, Paganelli C, et al. Randomized Clinical Trial on Short-Time Compression with Kaolin-Filled Pad: A New Strategy to Avoid Early Bleeding and Subacute Radial Artery Occlusion after Percutaneous Coronary Intervention. J Interv Cardiol 2011;24:65-72. [Crossref] [PubMed]
- Pancholy SB. Impact of two different hemostatic devices on radial artery outcomes after transradial catheterization. J Invasive Cardiol 2009;21:101-4. [PubMed]
- Zankl AR, Andrassy M, Volz C, et al. Radial artery thrombosis following transradial coronary angiography: incidence and rationale for treatment of symptomatic patients with low-molecular-weight heparins. Clin Res Cardiol 2010;99:841-7. [Crossref] [PubMed]


