Genesis on diamonds II: contact with diamond enhances human sperm performance by 300%
Introduction
Earlier we reported that the vitality of human sperm cells increased by 20% after 42 hours contact with nanocrystalline diamond coated Petri dishes, as compared to polystyrene Petri dishes. Herein we show that 1 hour contact with the nanodiamond Petri dishes enhanced the number of grade A motility human sperm cells (sperm cells that are swimming in a rapid progressive motility) by ~300%. Notably, the increase of the fraction of sperm cells with optimal motility was on cost of the low and non-motile sperm cells. This corresponds to an increase in overall sperm velocity on nanodiamond and is totally unexpected. One would expect a decrease in overall speed because the flagella propelled propagation should deplete the sperm’s energy reservoirs. Such a decrease is indeed observed on the polystyrene Petri dishes. The second result of the present study is that treatment with 670 nm light helps refueling the sperm’s depleted energy reservoirs. Recently, we showed that the unique biocompatibility of nanodiamond surfaces is due to the special structure of the nanoscopic interfacial water layers on top of them, and that the energizing effect of 670 nm light on cells is due to its effect on nanoscopic interfacial water layers in mitochondria. Earlier work suggested that the interplay between visible light and nanoscopic interfacial water layers on top of natural diamond surfaces played a fundamental role in the formation of the first polymers on Earth. The third result of this study, confirming previous data, is that polystyrene Petri dishes are detrimental for in vitro studies because they interfere with the cells, distorting the outcome of experimental results.
Previously, it was suggested that polystyrene Petri dishes soften when exposed to aqueous liquids, an effect facilitating the establishment of a nanoscopic layer of reactive oxygen species (ROS). This effect has been recognized in tests designed to quantify cell performance on polystyrene and on fully inert and ultrasmooth nanodiamond layers by the use of different cells as ROS-sensitive probes, including mouse embryonal carcinoma cells P19, murine fibrosarcoma cells L929 and human cervical cancer cells HeLa (1). The issue caught experts’ interest, as seen in the comments published by Nature Medicine (2): “Polystyrene Petri dishes may contribute to experimental error because the material can be prone to developing variable pH and changing surface hardness. Those two factors affecting cellular environment could also affect cellular behaviour”. The experimental proof of the nanomechanical softening has been provided in 2012 (3).
Pharmacological tests and tests designed to assess the biocompatibility of a new material conventionally start in the polystyrene Petri dish (in vitro), before animal tests and clinical studies follow. However, when it comes to in vitro fertilization (IVF) the Petri dish stands through the whole procedure, at the very beginning of human life. In 2013, we reported that about 20% more human sperm cells survived for 42 hours in diamond-coated Petri dishes than in the polystyrene dishes usually used for IVF (4). This circumstance and the aforementioned results were the principal motivation for the proposal to include nanodiamond coated Petri dishes into IVF routines. The detrimental effect of the polystyrene on sperm cells is ascribed to a superficial softening induced by aqueous liquids (buffered sperm culture medium) in combination with the establishment of a stable nanoscopic layer of ROS at the interface between the cells and polystyrene (4) which in turn increases interfacial viscosity by accentuating the hydrophilicity (5) of the polystyrene surface. By considering that an increase in interfacial viscosity corresponds to a decrease in the mobility of the molecules establishing the interface, it is clear that a high viscosity interfacial layer will tend to transiently entrap extra ROS molecules. Indeed, on hydrophilic surfaces interfacial viscosities of ten orders of magnitude above that of bulk liquid water have been reported (6). This means that ROS molecules, known to have short lifetimes in bulk liquid, where they are easily neutralized, experience an extension of their lifetimes on the surface of the polystyrene Petri dish (Figure 1)—a result which receives further confirmation by analogy with the lifetime extension of certain fluorescent dyes on surfaces.

In conjunction with the results of previous experiments carried out on nanodiamond surfaces and suggesting that the absolute chemical and biological innerness of this material is complemented by its capacity to bind a nanoscopic layer of water molecules to its surface, which in turn is acting as a viscoelastic layer determining the course of events of first contact between cells and diamond (8), we were led to assume that the nanodiamond surfaces are practically ROS-free. However, further tests indicated that nanodiamond Petri dishes are not only ROS-free in a passive sense but even have the capacity to actively neutralize the effects of ROS. In order to validate this hypothesis we measured the number of human sperm cells with motility grade A (per World Health Organization guidelines) after contact with polystyrene Petri dishes used in IVF and Petri dishes coated with nanodiamond. The parameter motility has been chosen because it reflects not only the level of the cell’s internal energy supply but also the impact of ROS. That ROS limits sperm cell motility is well known: ROS production in the cells involves electron leakage from mitochondria. The result of the mitochondrial ROS generation is damage to these organelles and initiation of an apoptotic cascade that leads to a decrease in sperm motility (9). We focused on sperm cells with rapid progressive motility (motility A) because it is believed that these are the cells with less chromosomal abnormalities and best DNA integrity.
Response of sperm cells to 670 nm light—on polystyrene and nanodiamond
Human sperm cells are particularly susceptible to ROS-induced damage because their plasma membranes contain large quantities of polyunsaturated fatty acids and their cytoplasm contains low concentrations of the scavenging enzymes. Therefore, many clinical and research institutes are investigating the usefulness of antioxidant supplementation in the prevention of the infertility problems. In vitro incubation under oxygen was found to be detrimental to human spermatozoa, decreasing motility and viability. Since then, many reports have associated ROS with impaired sperm function, including decreased motility, abnormal morphology, and decreased sperm-egg penetration (10). Many studies have demonstrated that low and physiological levels of ROS play an important role in processes such as capacitation, whereas high levels of ROS caused sperm pathologies including adenosine triphosphate (ATP) depletion with loss of motility and viability (11). One way to stimulate ROS production in mammalian cells, including sperm cells consists in their irradiation with red to near-infrared (NIR) light. While low doses of 670 nm light stimulate ATP synthesis in cells, probably by reducing the interfacial water viscosity within and around the mitochondrial rotary motor (12), elevated light doses are known to increase mitochondrial ROS production, which inhibits cellular functions as the ROS are spread all over the cell (13). The inhibitory effect can be understood by considering that during longer bombardment with ROS, the hydrophilic surfaces within and proximal to the mitochondrial rotary motor collect more ROS, which by increasing hydrophilicity, enhances interfacial water viscosity. Recent work supported the hypothesis that the root cause for the entire spectrum of biostimulatory effects (in which red to NIR light irradiation results in an increase in mitochondrial ATP synthesis) is a viscosity effect affecting the efficiency of the ATP synthase (14,15). For lower doses, corresponding to shorter irradiation times, the inhibitory effect of ROS is negligible due to the typically short lifetimes of ROS. In accordance with the shorter life times, effects of ROS vanish for shorter irradiation times because the number of molecules which prevail in an excited state is smaller; accordingly, while diffusing from the site of their generation, less molecules are likely to reach the relevant surfaces and interfaces in a reactive state. However, longer irradiation times [at biostimulatory intensities (16)] are associated with an extension of the lifetime of ROS due to the aforementioned transient immobilization of the ROS molecules at surfaces and interfaces. The amply documented increase in ATP synthesis in vitro in response to biostimulatory levels of red to NIR light, which is the predominant mechanism in low level laser therapy (LLLT), is in agreement with the following observation “Weaker initial ATP synthesis results in a higher positive laser effect” (17). These results can be derived from laboratory experiments (12,18) showing that the same light which increased ATP levels in cells is instrumental in reducing the viscosity of interfacial water. The interplay of light with nanoscopic interfacial water layers on diamond surfaces seems to play a fundamental role in nature: Together with diurnal light variations it explains the emergence of bioorganic molecules under primitive Earth conditions, which is one of the major unsolved origin of life questions. Accordingly, diamonds are the best of all possible origin of life platforms because they promote the formation of a crystalline interfacial layer of polarized water molecules on their surfaces, which imposes order to molecules (amino acids) landing on such surfaces—precondition for the formation of biopolymers (19).
Figure 2 illustrates the ROS scavenging/neutralizing effect of the nanodiamond on human sperm cells. According to current views ATP production in human sperm cells depends predominantly on the glycolytic pathway which produces less ROS than oxidative phosphorylation. The latter pathway seems to be predominant in stallion sperm ATP synthesis (21). To our knowledge, the glycolytic ATP synthesis pathway is not enhanced by red to NIR light irradiation, leading us to conclude that the increase in sperm motility observed 30 min after irradiation with red to NIR light (central wavelength 670 nm) delivered by an array of light emitting diodes (LED) was due to the interaction of the light with sperm mitochondria.
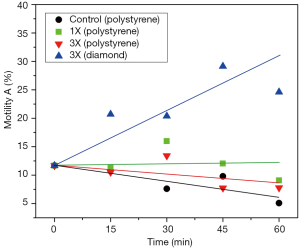
Nanodiamond for IVF
The number of grade A motility sperm cells measured on polystyrene Petri dishes (used in IVF) was enhanced by ~300% after 1 hour contact with the nanodiamond coated quartz Petri dishes. The results are statistically significant. An important result of the present study is the capability of relatively short light exposures to enhance sperm motility on polystyrene, whereas longer exposures showed an inhibitory effect. Given this, we hypothesize that the primary targets of the light are the mitochondrial rotary motors, and that the decrease in viscous friction enables them to produce extra amounts of ATP. The motors are propelled by the flow of protons that cross the mitochondrial membrane. However, the most striking result of the present study is the difference in sperm motility after contact with polystyrene and nanodiamond for prolonged light exposure: In combination with polystyrene, a light dose of 3X is clearly detrimental to sperm motility, reducing its levels to those of the control group, in particular after 45 min and 60 min. On the other hand, when in combination with nanodiamond, the exposure to the same light dose resulted in a massive increase in progressive motility. Figure 3 has been included as a visual synopsis of the antagonistic effects between mitochondrial ATP synthesis and ROS generation.
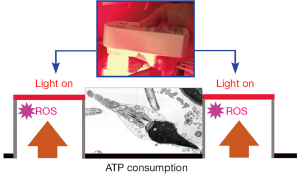
Further consideration of the aforementioned interplay between the lifetime of ROS and synthesis of ATP leads us to propose and justify the use of the pulsed irradiation mode in IVF, particularly under conditions of oxidative stress. The process of pulse optimization involves the experimental evaluation of two parameters: the period of time needed for light of a certain wavelength and intensity to elevate the mitochondrial ATP levels in a well defined environment, and the period of time in which the cells exhaust the ATP stores. This approach, proposed by us in recent paper (22), was experimentally confirmed in a boar sperm model (23). Once the individual parameters are known it is possible to adjust the duration of the light pulse and the dark period between pulses to a balanced ratio accounting for the specific energy demand of the cell. Extending the duration of the light pulse means extra oxidative stress due to ROS generation—extending the dark period beyond a critical limit (ATP deprivation) is expected to reduce cell viability (24). Concomitance of both extremes could result in a synergistic effect irreversibly damaging oxidatively stressed cells. It is instructive to explain the results shown in Figure 2 on the basis of Figure 3: obviously, there are two, in principle independent possibilities to increase the performance of sperm cells. The first is simply contact with the nanodiamond surface, the second consists in the administration of a light dose which is known to increase mitochondrial ATP levels (green line in Figure 2). Combining the two possibilities by using an intermittent irradiation mode adjusted to the sequence between ATP consumption and the time required to charge the sperm cell’s ATP reservoirs could help to further maximize the performance of the sperm cells. Considering that the polystyrene Petri dish, the temporary home to embryos growing in the laboratory, is used today in more than 99.9% of IVF procedures (25), the necessity for additional research is clear.
Acknowledgements
AP Sommer thanks Prof. Richard G. Rawlins for continuing support of the project.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sommer AP, Zhu D, Scharnweber T, et al. On the social behaviour of cells. J Bionic Eng 2010;7:1-5. [Crossref]
- News in Brief. Nature Medicine 2010;16:503.
- Sommer AP, Haddad MKh, Fecht HJ. It is Time for a Change: Petri Dishes Weaken Cells. J Bionic Eng 2010;9:353-7. [Crossref]
- Sommer AP, Zhu D, Gagsteiger F, et al. Sperm performance better on diamond than on polystyrene. Online Proceedings Library of the Materials Research Society 2013;1511.
- Ortiz-Young D, Chiu HC, Kim S, et al. The interplay between apparent viscosity and wettability in nanoconfined water. Nat Commun 2013;4:2482. [Crossref] [PubMed]
- Jinesh KB, Frenken JW. Capillary condensation in atomic scale friction: how water acts like a glue. Phys Rev Lett 2006;96:166103. [Crossref] [PubMed]
- Random Sample. Diamonds are sperm's best friend. Science 2013;339:744.
- Sommer AP, Zhu D, Franke RP, et al. Biomimetics: Learning from diamonds. J Materials Research 2008;23:3148-52. [Crossref]
- Aitken RJ, Jones KT, Robertson SA. Reactive oxygen species and sperm function--in sickness and in health. J Androl 2012;33:1096-106. [Crossref] [PubMed]
- Tafuri S, Ciani F, Iorio EL, et al. Reactive Oxygen Species (ROS) and Male Fertility. In: Wu B. editor. New Discoveries in Embryology. InTech, 2015:19-33.
- Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl 2002;23:737-52. [PubMed]
- Sommer AP, Haddad MKh, Fecht HJ. Light Effect on Water Viscosity: Implication for ATP Biosynthesis. Sci Rep 2015;5:12029. [Crossref] [PubMed]
- Lavi R, Shainberg A, Friedmann H, et al. Low energy visible light induces reactive oxygen species generation and stimulates an increase of intracellular calcium concentration in cardiac cells. J Biol Chem 2003;278:40917-22. [Crossref] [PubMed]
- Quirk BJ, Whelan HT. Effect of Red-to-Near Infrared Light on the Reaction of Isolated Cytochrome c Oxidase with Cytochrome c. Photomed Laser Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Al Musawi MS, Jaafar MS, Al-Gailani B, et al. Erythrocyte sedimentation rate of human blood exposed to low-level laser. Lasers Med Sci 2016;31:1195-201. [Crossref] [PubMed]
- Sommer AP, Pinheiro AL, Mester AR, et al. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA's light-emitting diode array system. J Clin Laser Med Surg 2001;19:29-33. [Crossref] [PubMed]
- Mester A. Laser biostimulation. Photomed Laser Surg 2013;31:237-9. [Crossref] [PubMed]
- Sommer AP, Haddad MKh, Fecht HJ. Tuning the wheel of life with light. In: Proceedings of the International Conference on Laser Applications in Life Sciences. Ulm, Germany, 2014:145.
- Sommer AP, Zhu D, Fecht HJ. Genesis on Diamonds. Cryst. Growth Des 2008;8:2628-9. [Crossref]
- Sommer AP, Zhu D. Facial rejuvenation in the Triangle of ROS. Cryst Growth Des 2009;9:4250-4. [Crossref]
- Gibb Z, Aitken RJ. The Impact of Sperm Metabolism during In Vitro Storage: The Stallion as a Model. Biomed Res Int 2016;2016:9380609.
- Sommer AP, Mester AR, Trelles MA. Tuning the mitochondrial rotary motor with light. Ann Transl Med 2015;3:346. [PubMed]
- Yeste M, Codony F, Estrada E, et al. Specific LED-based red light photo-stimulation procedures improve overall sperm function and reproductive performance of boar ejaculates. Sci Rep 2016;6:22569. [Crossref] [PubMed]
- Tian J, Zeng X, Xie X, et al. Intracellular Adenosine Triphosphate Deprivation through Lanthanide-Doped Nanoparticles. J Am Chem Soc 2015;137:6550-8. [Crossref] [PubMed]
- Cohen J. Growing Embryos in a World That is Flat: A Short History of Embryo Culture in Petri Dishes. Fertility Magazine 2006;6:12-24.


