Liquid biopsy is a rapidly emerging technique that measures extracellular DNA in a patient’s blood and is of considerable interest as a non-invasive method for early recognition and management of cancer.1,2 Circulating cell-free DNA (cfDNA) fragments in the blood are present at low levels in healthy individuals and at higher levels in patients with clinical disorders such as stroke, myocardial infarction, inflammatory or autoimmune disorders, and cancer.3,4 Most cancers have somatic mutations and epigenetic modifications which lead to activation, progression and metastasis of tumors.5,6 Such mutations include single-nucleotide base substitutions, insertions, deletions, gene fusions, gene amplification, losses of heterozygosity, and methylation changes.5 Circulating DNA originating from tumors, referred to as circulating tumor DNA (ctDNA), is a component of cfDNA,7 and the mutations present in ctDNA have high concordance with those present in tumor tissue biopsies.8 The rate of ctDNA shedding into blood has been shown to depend on tumor type, location, size, stage, and therapeutic response.9–11 Detection of circulating tumor cells (CTCs) is another means by which liquid biopsies can be used to detect cancer and to allow the mutational profile to be characterized without the need for direct cancer tissue biopsy.12
Endocrinologists rely on serum or plasma levels of protein or steroid hormones and other markers to diagnose and monitor functional endocrine neoplasms. However, many endocrine tumors are nonfunctional or secrete hormones or proteins that do not cause clinical syndromes (e.g. calcitonin in medullary thyroid carcinoma, thyroglobulin in differentiated thyroid carcinoma, glycoprotein alpha subunit or pancreatic polypeptide in islet cell tumors). Additionally, many endocrine glands are associated with a high prevalence of nonfunctional “incidentalomas”. A means to differentiate between benign and malignant lesions through a blood test without the need for a tissue biopsy would be desirable as such a test would be less costly and carry lower morbidity than invasive procedures.
The analytical methods available for measuring these components in the blood are numerous and rapidly advancing13,14 bringing liquid biopsy closer to clinical utility. Therefore, we reviewed the current status of liquid biopsies for diagnosis and management of endocrine neoplasms, specifically those involving the adrenals, thyroid, ovaries, and testes as well as pancreatic neuroendocrine tumors (NETs). We were unable to locate any studies evaluating liquid biopsy in patients with pituitary or parathyroid neoplasms.
Methods
We conducted systematic searches on PubMed and Embase databases between September through November 2018. We chose a 5-year timeframe (2014–2018) to provide a summary of the recent advances in liquid biopsy biomarkers.
For a comprehensive list of publications in each database, search terms for liquid biopsy, “cell-free DNA” or “cfDNA”, “circulating tumor cells” or “CTCs”, “circulating tumor DNA” or “ctDNA” were used. A broad search was conducted for endocrine neoplasia which included endocrine neoplasia, endocrine tumors, or endocrine cancer. The search was then expanded to search under individual endocrine organ terms for “adrenal tumors”, “ovarian endocrine tumors”, “pancreatic islet cell tumors”, “pancreatic neuroendocrine tumors”, “parathyroid tumors”, “pituitary tumors”, “testicular tumors”, and “thyroid tumors”.
Two reviewers conducted the screening. From the database search results, abstracts were first reviewed for studies that included serum or plasma samples from patients with endocrine cancer and reported analysis of CTC, cfDNA, or ctDNA. Studies which included other types of biomarkers, such as microRNA, in addition to CTC, cfDNA, or ctDNA were included. Studies assessing patients with several cancer types were included if at least one type of cancer studied was of endocrine origin.
Those featuring original data relating to absolute numbers in regard to detection of circulating biomarkers using a liquid biopsy for diagnosis and/or treatment monitoring in patients with endocrine cancer were retained for assessment. Articles cited in original reports and review articles identified in the database search were added to the review. Comments, letters, editorials and expert opinions, and abstracts that did not specify quantitative results were excluded. Articles including blood samples from adult human study populations were retained for review (publications of adrenocortical tumors in pediatric patients were excluded during the screening). Studies that investigated only tumor tissue, cell lines, and lavage fluid DNA with no liquid biopsy sample as part of the study were excluded. Some studies that measured tumor RNA in serum or plasma were included.
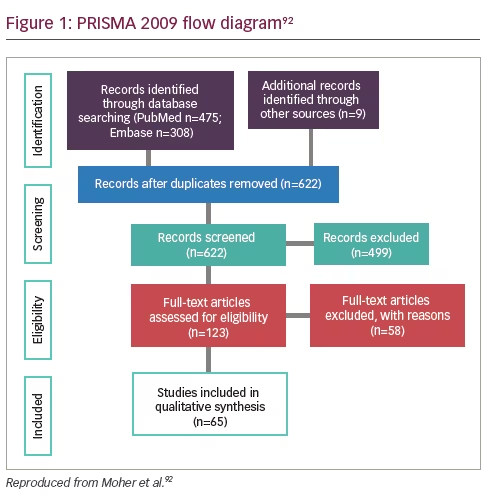
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart, the relevant manuscripts were selected for full-text review (Figure 1). Data extracted from each full-text publication or abstract were as follows: authors and publication year, endocrine organ, biomarker type, genes targeted, cases and controls (if applicable), time of sampling, study aims, and results. For study results, our primary outcome of interest was the prevalence of detection and/or estimate of diagnostic accuracy and area under the curve (AUC) cut-off values, sensitivity, and specificity as well as prevalence by cancer stage. We calculated sensitivities and specificities if these were not specifically determined and the manuscripts included sufficient individual patient results.
Results
A total of 622 unique publications were assessed for eligibility. Of those, 123 full-text articles were reviewed and 65 publications were identified for inclusion (Figure 1). Of these, 16 were abstracts that report quantitative data and the remaining were peer-reviewed manuscripts. The primary reason studies were excluded was due to not evaluating patients with endocrine neoplasia. For example, most of the ovarian cancer articles studied epithelial ovarian cancer subtypes and we aimed to assess germ cell or sex-cord tumors. Recent reviews of liquid biopsies and epithelial ovarian cancers were identified in our search.15–21
Some studies in patients with ovarian cancer did not report histopathology, and we included these in this review with indication of ovarian cancer type not specified. Similarly, we excluded studies that assessed liquid
biopsies only in patients with pancreatic ductal adenocarcinoma and restricted our results to studies that included patients with pancreatic NETs (pNETs), bronchopulmonary NETs (BPNETs), and gastroenteropancreatic NETs (GEPNETs).
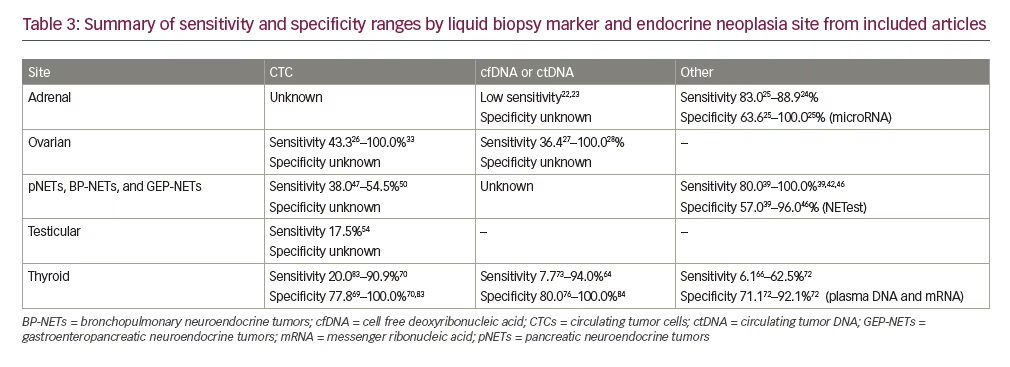
By cancer type, the majority of publications studied liquid biopsies in thyroid cancer (n=32) followed by endocrine ovarian cancer (n=13) and NETs (n=12). Four papers included patients with adrenal and testicular cancers. No publications were identified in our search for parathyroid and pituitary neoplasms. Table 122–54 summarizes each publication categorized by tumor site (adrenal, ovarian, NETs, and testicular) and Table 255–85 includes thyroid cancer publications. Table 3 summarizes the sensitivities and specificities for CTCs, cfDNA/ctDNA, and other biomarkers by endocrine tumor site.
Adrenocortical carcinomas
Circulating tumor cells
No data available
Circulating cell-free DNA or circulating tumor DNA
Assays measuring mutations in cfDNA22 and ctDNA23 showed sensitivities of 33.0% and 25.0%, respectively for detecting the mutations in plasma that were present in the primary tumor samples. Though limited, findings from these studies suggest metastatic adrenocortical carcinomas secrete ctDNA, and when detected, the ctDNA mutations followed tumor dynamics. However, Creemers et al.22 were unable to detect mutations in the cfDNA from two of three patients with known cancer tissue. Similarly, ctDNA was not detected in some patients with progressive disease with large tumor burdens.23
Other
Perge et al.24 and Salvianti et al.25 measured levels of microRNAs in plasma. Both miR483 and miR483-5p plasma levels showed relatively high sensitivities (83.0–88.9%) and specificities (63.6–100.0%) at varying cut-off values (Table 122–54). Salvianti et al. also isolated CTCs in a small subset (n=13) of adrenocortical carcinoma patient samples which showed a significant positive correlation between CTC count and microRNA marker levels.25
Ovarian endocrine tumors
Circulating tumor cells
Three studies measuring expression of epithelial cell adhesion molecule (EpCAM) in CTCs showed varying results.26,30,33 No CTCs were detected in two patients with stromal ovarian tumors studied by Lou and co-workers.30 Banys-Paluchowski et al.26 reported a detection prevalence of 43.3% (26/60) and Obermayr et al. reported that all patients with ovarian cancer were CTC-positive.33
Circulating cell-free DNA or circulating tumor DNA
Seven studies measured variant detection in ctDNA.27–29,31,35,37,38 In studies that included both tumor tissue-based biopsy mutation detection and ctDNA, ctDNA was detected at a lower frequency than tumor tissue.27–30,34–37 Wang et al. studied the use of a novel assay with the Papanicolaou (Pap) test fluid to increase sensitivity for ovarian cancers and reported that ctDNA was found in 43.0% of the patients in a plasma sample alone and when plasma and Pap brush samples were both tested, the sensitivity for ovarian cancer increased to 63.0%.37 Two patients with endocrine ovarian cancer were included in this study, but plasma samples were not taken in either of these samples. Both patients had negative Pap and Tao brush results and were negative for somatic and aneuploidy using the assay. However, the patient with a sex cord-like tumor had KRASand PIK3CA mutations identified in the primary tumor tissue.37 Detection of germline and somatic mutations in ctDNA in three of five endocrine ovarian cancers were observed,34 as well as in copy number alterations in a patient with dysgerminoma.32 One study did not find a significant difference between cfDNA in two patients with teratomas compared to healthy controls.36
Pancreatic, bronchopulmonary, and gastroenteropancreatic neuroendocrine tumors
Circulating tumor cells
Two studies reported a CTC detection rate between 38.0–54.5% in patients with pNETs using the CellSearch® assay (Menarini Silicon Biosystems, Inc., Huntington Valley, Pennsylvania, USA).47,50
Circulating cell-free DNA or circulating tumor DNA
In a case study, Wolff et al. mapped the disease progression and response to treatment including 15 cfDNA liquid biopsies measuring KRAS mutant allele fractions over a 5-year period in a patient with pancreatic ductal carcinoma with development of NET features, showing potential utility for monitoring tumor response during and following treatment.49Sikora et al. found overlap of cfDNA Alu83 and Alu244 levels between patients with pNETs, pancreatitis, and healthy controls; no significant association between tumor size or other variables was observed in patients with pNETs, indicating nonspecificity of the test.48
Other
Most of the studies identified in the search evaluated NETs using the NETest® assay (Wren Laboratories, Branford, Connecticut, USA). The NETest is a multigene assay targeting 51 genes with an algorithmic analysis from isolated microRNA and provides a score of disease status ranging from 0–100%.86 Determination of various NETest scores were reported with sensitivities between 80.0–100.0%, specificities ranging from 57.0–96.0%, and diagnostic accuracy AUC values between 0.81–0.98.39–42,44–46 In the studies that compared NETest scores with chromogranin A levels, the NETest assay was a more sensitive and specific biomarker.40,41,45,46 The assay also differentiated progressive from stable disease status.39,40,42–44
Testicular cancer
Circulating tumor cells
Of the four studies in patients with testicular cancer, most51,52,54 evaluated detection of CTCs as predictive of clinical response and progression. The largest study included 143 patients with germ cell tumors, of which CTCs were detected in 17.5% (25/143) of blood samples using Ficoll density gradient centrifugation, compared to 11.5% (14/122) of blood samples using the CellSearch assay.54 The presence of CTCs significantly correlated with tumor histology, stage of disease, and tumor marker levels in blood serum in these patients.54
Circulating cell-free DNA or circulating tumor DNA
No data available.
Other
A study identified 20 candidate genes in microRNA of patients with seminoma using microarray analysis by measuring copy number before and after surgery.53
Thyroid cancer
Circulating tumor cells
Overall, 11 of the 32 studies measured CTCs in patients with thyroid cancer. A prospective study screened subjects with an increased risk of cancer and CTCs were detected in half (132/265) of the screened participants; of those, 24 subjects (20.0%) had cancer confirmed within the following year. Five additional known patients with thyroid cancer had detectable CTCs.78 Ehlers et al. reported a significant increase in CTCs in patients with thyroid cancer compared to the normal control group, but tumor free patients still had positive CTC results.62 CTC number correlated with initial tumor stage, and there was no difference in CTC number between follicular, papillary, or medullary thyroid cancer subtypes or metastatic status.62 CTC detection in patients with differentiated thyroid carcinoma ranged from 69.3%85 to 86.0%77 and one study reported a CTC detection rate of 72.0% in patients with medullary thyroid cancer.83 Xu and colleagues reported CTC marker accuracy using cut-off values of ≥5 CTCs and 1 CTC to distinguish patients with metastatic disease from controls. Sensitivities were 20.0% and 41.0% and reported specificities were 100.0% and 90.0%, respectively.83 Also using a cut-off ≥5 CTCs, a sensitivity of 64.3% and specificity of 83.8% was reported in predicting patients with differentiated thyroid carcinoma with distant metastasis.77 However, Winkens et al. found no correlation between disease status and changing levels of CTC before or after radioactive iodine therapy, and there was no significant correlation between thyroglobulin levels and the percent change of CTC at any time point in 28 patients with differentiated thyroid carcinoma.82
Sensitivities ranged from 72.2–90.0% and specificities were 92.3–100.0% using four different cut-off values for EpCAM and thyroid stimulating hormone receptor (TSHR) CTC markers to distinguish patients with distant metastasis from disease-free status.70 In a long-term follow-up study, Lin et al. reported accuracies of EpCAM, TSHR, and podoplanin markers between 77.3–80.4% in determining remission from non-remission patients and between 67.2–69.5% in distinguishing survival status.71 Using EpCAM and TSHR CTC markers to predict recurrence, EpCAM showed a sensitivity of 87.5% and specificity of 100.0%, and TSHR showed the same sensitivity but lower specificity (77.8%) in patients with papillary thyroid carcinoma.69 Measuring EpCAM, cytokeratins, thyroglobulin and sodium:iodide symporter, and CD45, Dent et al. reported an overall CTC detection in 66.7 (4/6) patients with thyroid cancer.61
Circulating cell-free DNA or circulating tumor DNA
Eighteen studies measured mutation detection in cfDNA or ctDNA. In a study measuring the APP gene integrity in cfDNA, the AUC values were between 0.765–0.982 when comparing healthy subjects to patients with a cytological diagnosis of thyroid carcinoma, and 0.699 when comparing patients with benign nodules to patients with thyroid carcinoma.79 In patients with thyroid nodules, Lupo et al. reported a sensitivity of 7.7% and specificity of 95.4% between detection of ctDNA and pathologic diagnosis and molecular testing from fine-needle aspiration biopsy results73 and Li et al. reported a sensitivity for thyroid cancer of 28.0% using 5hmC markers in cfDNA.68 In a large study in patients with various cancer types, ctDNA was detectable in less than 50.0% of thyroid cancers, however, the number of patients with thyroid cancer was small (n=4) and the subtype of cancer was not reported.9
Several studies aimed to detect BRAFV600E. Pupilli et al. evaluated BRAFV600E (Taqman® Assay, Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) mutated allele in cfDNA as a marker for the diagnosis and follow-up of papillary thyroid carcinoma which showed a sensitivity of 65.0% and specificity of 80.0% for diagnostic performance and circulating BRAFV600E showed a significantly lower percentage in cfDNA 3–6 months after surgery.76 In a study measuring BRAFV600E to distinguish between benign and malignant thyroid nodules, all patients with detectable BRAFV600E ctDNA (14.8%; 9/61) had classical papillary thyroid carcinomas and none of the benign thyroid nodules had detectable BRAFV600E in the preoperative sample.74
In patients with advanced thyroid cancers two studies reported no BRAFV600E mutations from ctDNA samples with BRAFV600E positive tissue samples.59,84 In patients with advanced papillary thyroid carcinoma, Kim et al. reported a 6.1% detection rate in plasma DNA in samples known to harbor the BRAF mutation in tissue, and all patients with positive plasma BRAF mutation were stage IVC.66 Janku et al. reported higher concordance with detectable ctDNA in 50.0% (5/10) of patients with BRAFV600E positive tissue,65 and Iyer et al. found 91.0% concordance between BRAF mutation detection and tissue, with a reported sensitivity of 94.0% and specificity of 89.0% in patients with anaplastic thyroid cancer.64 Of note, in a study comparing detection of BRAFV600E mutation in ctDNA to imaging in patients with BRAF-positive anaplastic thyroid cancer, concordance was 94.0–100.0% in patients with regression and stable disease but only 47.0% in patients with progressive disease.63 All patients with radioactive iodine refractory differentiated thyroid carcinoma with BRAFV600E mutation in ctDNA at baseline had undetectable levels after three dabrafenib ± trametinib treatment cycles.67
A multigene panel reported an overall 67.0% ctDNA detection rate, which was highest in patients with metastatic disease (79.0%) compared to local recurrence (33.0%) or no macroscopic disease (0%).56 In a 70-gene assay (Guardant360®, Guardant Health Inc., Redwood City, California, USA), overall concordance was 72.0% in 12 patients with anaplastic thyroid cancer without treatment but 100.0% concordance for the presence of BRAFV600E and NRAS mutations.80 Patients with surgery or treatment prior to study (n=7) had 6.0% concordance and there was no concordance between tissue and ctDNA in patients without active disease at the time of the study.80 In patients with advanced malignancy, the Guardant360 assay had high specificity, but low sensitivity, with a diagnostic accuracy range of 82.0–89.0% for concordance of genomic alterations obtained from tissue biopsies and plasma cfDNA.58 Lastly, a retrospective study measured ctDNA using the Guardant360 assay in 88 patients with head and neck squamous cell carcinoma in which TP53and PIK3CA were the most common mutations identified, and 75.0% of patients with thyroid cancer had actionable mutations.75
Studies which measured RET M918T mutation in ctDNA in patients with medullary thyroid cancer, reported 32.0%60 and 61.5%57 mutation detection in positive tissue mutation samples with a calculated 34.5% sensitivity and 84.2% specificity for distinguishing distant metastases.60 Busaidy et al. reported emergence of RET V804M mutation after tyrosine kinase inhibitor exposure.57
Other
A study measuring circulating tumor-associated microparticles (taMPs) included 43 patients with thyroid nodules and found EpCAM + taMPs significantly increased in this group compared to healthy controls.81 Using an assay measuring TSHR microRNA, Aliyev et al. reported no significant difference between positive and negative groups in the prevalence of lymph node metastases, or tumor size in 152 patients with papillary thyroid microcarcinoma.55 Lastly, Lubitz et al. compared circulating RNA BRAFV600E levels with BRAF mutation DNA-based tissue assays in patients with papillary thyroid carcinoma and reported sensitivities and specificities ranging from 43.8–62.5% and 71.1–92.1%, respectively, from three cut-off values.72
Discussion
In this review, we aimed to provide estimates of sensitivity and specificity of CTCs and circulating cell-free nucleic acids in detecting various endocrine neoplasms. There is interest in the use of liquid biopsies to assess tumor presence, disease progression, and monitoring of treatment efficacy. Compared to standard tissue biopsy, liquid biopsy offers a non-invasive method for determining tumor genotype profiling and genetic heterogeneity. Moreover, ctDNA detection technologies are highly sensitive, with only a few copies of mutant ctDNA needed for analysis.7,87 This is especially important for detection of early stage cancers of unknown primary, particularly because the prognosis is dependent of the stage at diagnosis. A screening test with tumor-specific markers with high sensitivity and specificity, and a high positive predictive value could reduce costs associated with cancer detection and improve patient outcomes. Liquid biopsies could also allow for longitudinal assessment of disease progression and response to treatment.
A common approach for assessing liquid biopsies has been to compare concordance between the detection of the analyte in the blood and detection in the tumor-based biopsy. Concordance of mutations found in tumor tissue and cfDNA/ctDNA varied greatly in many studies, indicating that a negative blood test does not indicate absence of tumor or absence of mutation in the tumor. It is unclear, however, whether the discordance is caused by different analytical sensitivity or confounded by biologic factors such as tumor type and stage, tumor heterogeneity, and time between blood sampling and tissue biopsy.88 CtDNA detection is increased in higher stage cancers, especially when comparing metastatic to localized disease.9 In patients with adrenocortical carcinomas, ctDNA was not detected in some patients with progressive disease with large tumor burdens,23 and it remains unclear whether ctDNA detection was negative due to assay sensitivity limitations or if adrenocortical carcinoma does not shed large quantities of ctDNA. Correspondingly, somatic variants detected in ctDNA may not be released from a tumor, but rather originate from clonal hematopoiesis.4,88–91 Increased levels of cfDNA are found in individuals with inflammatory or benign conditions, healthy individuals, and associated with aging,4,91 which may result in false-positive cases with ctDNA detection.
There were few studies identified for adrenal and testicular neoplasms, which limits the comparisons that can be made. Additionally, sample sizes were generally low. Of the three studies measuring CTCs in patients with testicular cancer, two are case reports.51,52 Due to the small number of patients in the majority of the clinical studies included in our analysis, especially when stratified by endocrine tumor type and stage, it is difficult to determine if assay sensitivities are useful for earlier or late stages or if there is no difference. For adrenal neoplasms, there is low sensitivity for differentiating benign adrenocortical adenoma from adrenocortical carcinoma using ctDNA,22,23 but microRNAs may be useful for monitoring therapy response in both high- and low-risk patients.25 Of the thirteen studies identified in our search studying endocrine ovarian cancer, five did not specify histopathology,26–29,33,35 which further limits the determination of clinical utility. In those that distinguished cancer type, between two and five patients per study with endocrine ovarian cancer diagnosis were evaluated with varying results.30,32,34,36,37 Studies evaluating the NETest assay in NETs reported high sensitivities between 80.0–100.0% and specificities between 57.0–96.0%.39,40,45,46 However, there is variability in cut-off values, inclusion of a control group, and timing of blood and tumor samples in these studies. Larger prospective studies could increase the ability to determine the clinical utility of NETest in well-differentiated pancreatic endocrine neoplasms and intestinal and pulmonary carcinoids.
The thyroid cancer articles differed widely by study methodology and the proportion of various histological subtypes which could account for the wide sensitivity measurements. Thus, there is limited evidence to support the use of liquid biopsies over fine-needle aspiration biopsy to differentiate between patients with an indolent tumor from those with aggressive tumors.61,62,66,68,73,74,78,79,81,84 There are also limited data on treatment monitoring in advanced thyroid cancers67,76,82,83,85 and how liquid biopsies ultimately compare to thyroglobulin for differentiated thyroid carcinoma, or calcitonin and carcinoembryonic antigen for medullary thyroid cancer, and imaging for monitoring.56,82 It is unclear how liquid biopsies compare to assays for tumor-associated proteins in other endocrine neoplasms as well. For example, serum ctDNA samples did not show a significant difference between patients with granulosa cell tumor and healthy controls, yet cancer antigen 125 (CA125) levels were significantly different.38 In contrast, the NETest assay consistently outperformed the chromogranin A test in patients with carcinoid tumors.40,41,45,46
Conclusion
Among the studies summarized in this analysis, there is a large variation in mutation detection and concordance with mutations found in tumor tissue (Table 3). Overall, the research to date does not support the use of liquid biopsy as a surrogate to tissue-based biopsy. However, some assays, such as the NETest for NETs, may be useful as an adjunct to other tests for diagnosis; and CTC, cfDNA, and ctDNA markers may have some utility to follow the clinical course once a tumor is diagnosed and the somatic mutations in the neoplasm identified.