Non-alcoholic fatty liver disease (NAFLD) is the most frequent cause of chronic liver disease globally; it is histologically classified into non-alcoholic fatty liver (NAFL) and non-alcoholic steatohepatitis (NASH).1 NAFL is characterized by the presence of >5% of hepatic steatosis without evidence of hepatocellular injury in the form of hepatocyte ballooning. NASH is the advanced stage of NAFLD comprising >5% of hepatic steatosis and inflammation, along with hepatocyte injury.2 If not treated in the early stage, NAFL can progress to NASH, hepatic fibrosis and cirrhosis. It has been recognized as the most frequent indication for hepatic transplantation.3 Furthermore, it is one of the leading causes of hepatocellular carcinoma.4
The risk of NAFLD increases with the presence of any component of metabolic syndrome, such as obesity, hypertension, high cholesterol and type 2 diabetes mellitus.5 Considering the importance of metabolic derangements and their impact on the pathogenesis of NAFLD, new nomenclature has been proposed for this disease entity: metabolic associated fatty liver disease (MAFLD).6,7 The need to rename NAFLD to MAFLD was highlighted by a group of researchers (comprised of an international consensus panel) from the Westmead Institute for Medical Research since they felt that the new terminology will define its aetiology in a clearer way and improve public health initiatives.7 Hence, the term NAFLD is likely to be replaced by MAFLD. The worldwide prevalence of NAFLD is estimated to be 25%.8 In western countries, overall prevalence of NAFLD is 15–40%, whereas epidemiological studies suggest the prevalence in the Indian population to be around 9–32%, with a greater incidence in patients suffering from obesity and diabetes.9
Epidemiological studies have shown an increased mortality with advanced fibrosis, which demands the urgent requirement of effective pharmacotherapy in patients with NAFLD.9 Considering the epidemic increase in the prevalence of NAFLD, its associated morbidity and mortality, and its rising burden of healthcare, there is a dire need to develop effective pharmacological approaches. This review will focus on the potential role of dexamphetamine in the treatment of NAFLD.
Current approaches for management of non-alcoholic fatty liver disease
There are no approved pharmacotherapeutic options currently available for the treatment of NAFLD.10 However, currently available treatment strategies for NAFLD consist of weight reduction, lifestyle modifications and treatment of the components of metabolic syndrome.10 In various randomized controlled trials, weight reduction has shown to improve liver enzymes, histology and insulin levels in patients with NAFLD, and it has been suggested that weight loss is the key factor to improve liver histology in patients suffering from NASH.11–13 There is some supporting evidence regarding the use of insulin sensitizers, like pioglitazone and vitamin E; however, their use is not considered in all patients.14 Pharmacological treatment is typically reserved for patients who have failed to reach their weight-loss goal and those with biopsy-proven NASH with fibrosis stage ≥2.13 Trials addressing pharmacologic therapies have not been large or particularly informative enough to determine the impact of these therapies on important clinical outcomes, such as decompensated cirrhosis.13 Most of these trials addressed surrogate outcomes, such as serum aminotransferase levels or liver histology, and too often with conflicting results.13
In the guidelines provided by the American Association for the Study of Liver Disease and European Association for the Study of the Liver, a daily dose of 800 international units of vitamin E has been recommended for patients with NASH (biopsy-proven) without diabetes mellitus.2 Vitamin E has been shown to improve steatosis and inflammation, but it has not shown any effect on improvement of hepatic steatosis.2 However, a meta-analysis by Musso et al. revealed that there are no histologic benefits with the administration of vitamin E.13 However, this meta-analysis suffered from significant heterogeneity in terms of the formulations of vitamin E used, the patient participants, treatment duration and lifestyle modifications.13 Pioglitazone is also recommended for the treatment of patients with histology-proven NASH with or without diabetes mellitus, but its use is largely restricted because of its potential adverse effects.15 A meta-analysis by Li et al. in patients with NAFLD showed that metformin can improve body mass index (BMI), insulin resistance and hepatic transaminase levels, but does not improve steatosis and liver histology.16
Farnesoid X nuclear receptor (FXR) ligand, obeticholic acid, is being investigated for its potential role in the management of NAFLD.17 FXR has a prominent role in the synthesis and enterohepatic circulation of bile acids, and also regulates the lipoprotein, immune inflammatory and fibrotic pathways important in NAFLD. Obeticholic acid acts as an FXR agonist and it helps in correcting the abnormalities in cholesterol lipoprotein and bile acid metabolism, and modulates the immuno-inflammatory and fibrogenic changes seen in NAFLD. Obeticholic acid has shown promising results in clinical trials and has led to improvements in liver histology, insulin sensitivity, and has reduced markers of liver inflammation and fibrosis.18,19
Dexamphetamine in non-alcoholic fatty liver disease: potential role and current evidence
Dexamphetamine is the d-isomer of amphetamine, which is an indirectly-acting sympathomimetic drug, inhibiting reuptake of monoamines and direct stimulation of release of dopamine and noradrenaline.20 Dexamphetamine firstly enters the presynaptic neuron where it binds to the vesicular monoamine transporter-2. It prevents the translocation of monoamine from the cytosolic pool to storage vesicles, leading to intracellular monoamine increases. This also stimulates the amine-associated receptor intracellular trace1 to promote dopamine efflux. All these processes help to reverse the transportation through noradrenaline or dopamine transporter, enhancing extracellular monoamine release. Elevated release of monoamines promotes satiety and reduces feeding through the activation of postsynaptic α– and β-adrenergic receptors and D1/D2 receptors.21 The d-isomer of amphetamine is more potent than its I-isomer.22 It has an extensive distribution in most of the tissues and possesses a potent psycho-stimulant effect. After oral administration, a high concentration is achieved in the central nervous system, and it produces various effects, such as suppression of appetite and an improvement in concentration and cognitive functions.23 Currently, it is indicated for the treatment of attention deficit hyperactivity disorder and narcolepsy. Use of dexamphetamine was found to be associated with weight loss; hence, it is used as an off-label drug for the treatment of obesity.24,25
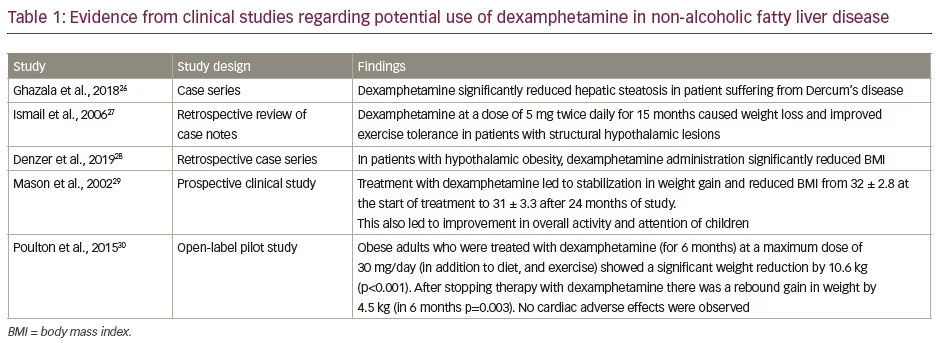
A case series published by Ghazala et al. revealed promising outcomes in two patients with Dercum’s disease.26 In case 1, in a 55-year-old man, administration of dexamphetamine for 3 months led to a reduction in weight and hepatic steatosis. Initially dexamphetamine was started at a dose of 10 mg but then reduced to 7.5 mg due to anxiety and jitteriness. Similar results were observed in case 2, a 52-year-old female with Dercum’s disease. The drug was initially started at a dose of 1.25 mg daily and was gradually increased to 20 mg daily. A year later magnetic resonance imaging also showed an improvement in hepatic steatosis.26 Although the case series does not carry a significant weight towards evidence-based medicine, the promising results obtained from this particular case series demand further clinical studies involving adequate sample size.
A retrospective study by Ismail et al. in patients with structural hypothalamic lesions revealed that dexamphetamine 5 mg twice daily for 15 months led to weight loss and improvement in exercise tolerance.27 Another retrospective case series study by Denzer et al. in seven patients suffering from hypothalamic obesity also led to promising results.28 In all patients, dexamphetamine was started at 5 mg per day and gradually increased to up to 20 mg per day. In all patients with hypothalamic obesity, a reduction in BMI was observed compared to baseline and no troublesome adverse effects were observed.28
Mason et al. performed a prospective study on five children who had a poor attention and history of significant weight gain following surgical treatment for craniopharyngioma.28 In this study, treatment with dexamphetamine was started at 5 mg and was increased gradually by 2.5 mg per week until adverse effects and decrease in appetite were observed. Dexamphetamine also stabilized the weight gain and reduced BMI from 32 ± 2.8 at the start of treatment to 31 ± 3.3 after 24 months of study. Administration of dexamphetamine also showed an improvement in overall activity and attention of children included in this study.28
In a pilot study by Poulton et al., the anorexigenic effect of dexamphetamine was evaluated for promoting weight loss.30 Obese adults were treated with dexamphetamine for 6 months at a maximum dose of 30 mg day in addition to diet and exercise. This approach led to a significant weight reduction of 10.6 kg (p<0.001). After stopping the therapy with dexamphetamine there was a rebound gain in weight of 4.5 kg (in 6 months p=0.003). In addition, no cardiac adverse events were observed. Hence, this study provided important preliminary evidence that dexamphetamine in addition to diet and exercise may offer a safe and effective approach for weight loss, and this may also be helpful in patients with NAFLD. Evidence from clinical studies has been summarized in Table 1.26–30
Indirect evidence
Although this section does not address the role of dexamphetamine per se, we have narrated the evidence obtained from related compounds with similar mechanisms to that of dexamphetamine. In a preclinical study by Decara et al. on obese Zucker rats, it was found that treatment with oleic acid and dihydroxyamphetamine, given for 15 days, reduced histologically-confirmed hepatic steatosis as well as plasma triglyceride levels.31
Sibutramine (a serotonin and norepinephrine reuptake inhibitor that acts by increasing energy expenditure and satiety), an older anti-obesity drug, has been shown to be effective in the treatment of NAFLD. In a study by Sabuncu et al. on obese patients suffering from NASH with mean BMI of 37.3 kg/m2, it was found that treatment with sibutramine for 6 months significantly reduced body weight, hepatic steatosis and insulin resistance. Improvement in levels of serum transaminases was also observed, except for alkaline phosphate.31 One randomized, double blind, placebo-controlled trial in 92 participants has been planned to evaluate the safety and tolerability of phentermine 15 mg for 6 weeks for the reduction of hepatic steatosis, adipose tissue and postoperative complications in patients undergoing bariatric surgery and those requiring bariatric surgery.33
Potential safety concerns of dexamphetamine
Dexamphetamine is well tolerated and is rarely associated with troublesome adverse events. Neurological adverse effects are mainly observed in patients suffering from previous psychiatric illnesses.34 Neuropsychiatric adverse effects caused by dexamphetamine include insomnia, anxiety, confusion and delusions. Other adverse effects include gastrointestinal events (nausea, abdominal discomfort) and retention of urine. Hypertension and cardiac arrhythmias can occur at higher doses, but they tend to resolve after dose reduction.21,35 A study by Williams et al. showed that dexamphetamine has been a less commonly abused drug compared to abuse of other substances.35 Apart from this, dexamphetamine can lead to some clinically significant drug interactions with drugs such monoamine oxidase inhibitors; antacids and other drugs used for peptic ulcers; antipsychotics, such as chlorpromazine and haloperidol; norepinephrine; and anticonvulsants, such as phenytoin and phenobarbital. As such, caution should be taken while prescribing dexamphetamine along with potentially interacting drugs.36,37
Future considerations
The prevalence of NAFLD is increasing day by day, and currently no effective pharmacotherapeutic options are available to address this issue.9 Published literature has shown the promising effects of dexamphetamine in hypothalamic obesity and NAFLD.26-30 Although dexamphetamine has some neuropsychiatric, gastrointestinal and cardiovascular adverse effects,21,34,35 the potential benefits may outweigh the risks. Introduction of the term MAFLD in place of NAFLD reflects the importance of metabolic parameters in the pathogenesis of this clinical entity.6,7 As BMI and weight, along with factors such as insulin resistance, play a crucial role in the development of MAFLD,5,6,7 dexamphetamine can play a crucial role in addressing these metabolic derangements. Studies assessing the preclinical effect of dexamphetamine in NAFLD are lacking; therefore, more preclinical studies are required to explore the potential effect of dexamphetamine for the treatment of NAFLD. In addition, there is a dire need for clinical studies involving robust methodology and adequate sample size to explore the potential of dexamphetamine in patients with NAFLD. This can also help in defining the dose range of dexamphetamine for this indication. However, from the available evidence, it can be said that dexamphetamine could emerge as a potential therapeutic option for the treatment of NAFLD.
Conclusion
Dexamphetamine appears to possess promising potential for the treatment of NAFLD. However, it has some safety concerns and further data are needed on its efficacy. To fulfil the unmet need of NAFLD treatment, there is a significant need to undertake research and generate a high level of evidence to ascertain its efficacy and safety for the treatment of NAFLD.