NANOSYSTEMS: PHYSICS, CHEMISTRY, MATHEMATICS, 2017, 8 (6), P. 830–834
The solubility of sodium and potassium fluorides in strontium fluoride
P. P. Fedorov – Prokhorov General Physics Institute, Russian Academy of Sciences, Moscow; Ogarev Mordovia State University, Saransk, 430005, Russia; ppfedorov@yandex.ru
M. N. Mayakova – Prokhorov General Physics Institute, Russian Academy of Sciences, Moscow, 119991, Russia
V. A. Maslov – Prokhorov General Physics Institute, Russian Academy of Sciences, Moscow, 119991, Russia
A. E. Baranchikov – Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Moscow, 119991, Russia
V. K. Ivanov – Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, Moscow, 119991, Russia
A. A. Pynenkov – Ogarev Mordovia State University, Saransk, 430005, Russia
M. A. Uslamina – Ogarev Mordovia State University, Saransk, 430005, Russia
K. N. Nishchev – Ogarev Mordovia State University, Saransk, 430005, Russia
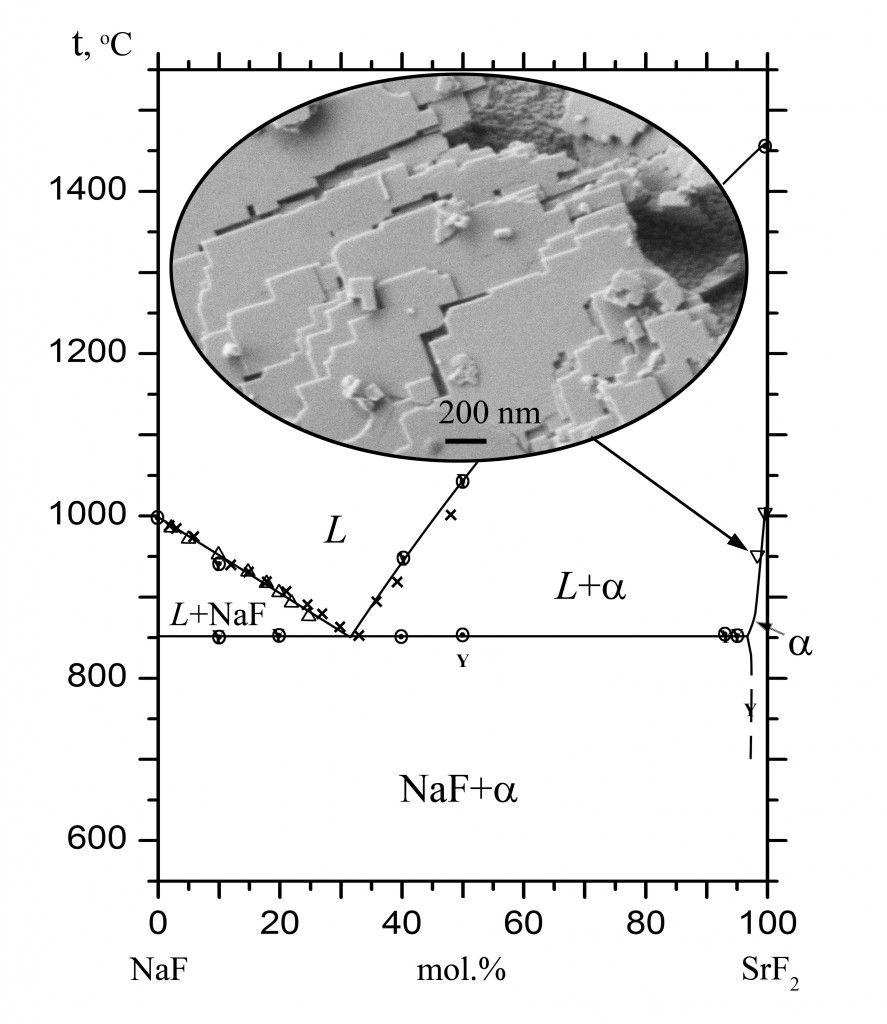
The phase diagram of the NaF–SrF2 system was studied by thermal analysis and X-ray powder diffraction analysis with the determination of the chemical composition. The system was found to be of the eutectic type. The eutectic coordinates are 853 °C, 32 mol % SrF2. A narrow range of the existence of solid solution Sr1-xNaxF2-x was established. The NaF solubility reaches a maximal value of x = 0.035 at eutectic temperature. The solubility of KF in SrF2 is very low.
Keywords: strontium fluoride, sodium fluoride, potassium fluoride, nanofluorides, NaF-SrF2 phase diagram, solid solution.
DOI 10.17586/2220-8054-2017-8-6-830-834