Abstract
Objective
Serogroup B meningococci (MnB) are now the largest cause of invasive meningococcal disease (IMD) in Canada. We assessed the clinical and economic impact of 3 adolescent MenB-FHbp immunization strategies.
Methods
A population-based dynamic transmission model was developed to simulate the transmission of MnB among the entire Canadian population over a 30-year time horizon. Age group-based IMD incidence, bacterial carriage and transmission, disease outcomes, costs, and impact on quality of life were obtained from Canadian surveillance data and published literature. The vaccine was assumed to provide 85% protection against IMD and 26.6% against carriage acquisition. The model estimated the impact of routine vaccination with MenB-FHbp in 3 strategies: (1) age 14, along with existing school-based programs, with 75% uptake; (2) age 17 with 75% uptake, assuming school vaccination; and (3) age 17 with 30% uptake, assuming vaccination outside of school. Costs were calculated from the Canadian societal perspective.
Results
With no vaccination, an estimated 3974 MnB cases would be expected over 30 years. Vaccination with strategies 1–3 were estimated to avert 688, 1033, and 575 cases, respectively. These outcomes were associated with incremental costs per quality-adjusted life-year of $976,000, $685,000, and $490,000.
Conclusions
Our model indicated that if the vaccine reduces risk of carriage acquisition, vaccination of older adolescents, even at lower uptake, could have a significant public health impact. Due to low disease incidence, MnB vaccination is unlikely to meet widely accepted cost-effectiveness thresholds, but evaluations of new programs should consider the overall benefits of the vaccination.
Résumé
Objectif
Le méningocoque du sérogroupe B (MnB) représente aujourd’hui la cause la plus importante de méningococcie invasive au Canada. Cette analyse a évalué les répercussions cliniques et économiques de 3 stratégies d’immunisation à l’aide du vaccin contre le MenB-fHBP chez des adolescents.
Méthodes
Un modèle dynamique de transmission dans la population a été créé afin de simuler la propagation du MnB dans l’ensemble de la population canadienne pendant une période de 30 ans. L’incidence de la méningococcie invasive par groupe d’âge, les taux de portage et de transmission bactérienne, l’évolution de la maladie, les coûts qui y sont associés et les répercussions sur la qualité de vie ont été tirés de rapports de surveillance canadiens et de la littérature. Il a été supposé que le vaccin offrait une protection contre la méningococcie invasive et contre l’acquisition du portage dans 85 % et 26,6 % des cas, respectivement. Le modèle étudié a évalué les répercussions associées à l’inoculation systématique par le vaccin contre le MenB-fHBP selon les 3 stratégies suivantes : (1) immunisation à l’âge de 14 ans, dans le cadre des programmes existants mis en œuvre en milieu scolaire, à un taux de vaccination de 75 %; (2) immunisation à l’âge de 17 ans, en milieu scolaire, à un taux de vaccination de 75 %; et (3) immunisation à l’âge de 17 ans, à un taux de vaccination de 30 %, en contexte extrascolaire. Les coûts ont été calculés en fonction de la perspective sociétale canadienne.
Résultats
Sans immunisation, le nombre estimé de cas de méningococcie du sérogroupe B serait de 3974 sur une période de 30 ans. Selon le modèle, les stratégies de vaccination 1 à 3 préviendraient respectivement 688, 1033 et 575 cas. Ces résultats ont été associés à des coûts différentiels par année de vie gagnée ajustée par la qualité (QALY) de 976 000 $, 685 000 $ et 490 000 $.
Conclusions
Le modèle utilisé dans le cadre de l’analyse a permis d’indiquer que si le vaccin réduisait le risque d’acquisition du portage, la vaccination des adolescents plus vieux, même en présence de taux de vaccination plus faibles, pouvait avoir des répercussions significatives sur la santé publique. En raison de la faible incidence de la maladie, il est peu probable que la vaccination contre le MnB respecte les seuils généralement acceptés en matière de coût-efficacité, mais l’évaluation de nouveaux programmes devrait tenir compte de l’ensemble des bienfaits associés à la vaccination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive meningococcal disease (IMD) is a rare but serious condition caused by Neisseria meningitidis, with potentially devastating consequences. The bacteria can be present on the nasopharyngeal mucosa without causing the disease but, in a small proportion of carriers, will invade the meninges or blood, leading to meningitis and/or septicemia (Crum-Cianflone and Sullivan 2016). The onset of IMD can be insidious and the early manifestations are often indistinguishable from a number of other mostly benign infections. IMD can be fatal within 24 h of onset (Public Health Agency of Canada 2015; World Health Organization 2018). Up to one third of survivors will have permanent severe sequelae such as hearing loss, neurological disabilities, or limb loss (Bettinger et al. 2013; Sadarangani et al. 2015; Public Health Agency of Canada 2015; Ontario Ministry of Health and Long-Term Care 2015). While disease incidence is highest in infants and children under 5 years of age, a significant number of cases occur in adolescents and young adults (National Advisory Committee on Immunization 2014)—the population segment in whom meningococcal carriage prevalence is highest (Christensen et al. 2010).
IMD is endemic in Canada and the majority of cases are caused by serogroup B (MnB) (National Advisory Committee on Immunization 2014; Li et al. 2014), including the recent outbreak in Nova Scotia, as well as the prolonged increased incidence in the province of Quebec (De Wals et al. 2017; Langley et al. 2016; Nova Scotia Department of Health and Wellness 2015). Serogroup B meningococcus was responsible for 63% of all IMD cases in Canada between 2011 and 2015 (Public Health Agency of Canada 2017). In October 2017, Health Canada approved the MenB-FHbp vaccine (Trumenba®, Pfizer) for use in individuals aged 10 through 25 years (Pfizer Canada Inc. 2018). Before then, the multicomponent MnB (4CMenB) vaccine (Bexsero®, Novartis), approved in December 2013, was the only MnB vaccine available in Canada (GlaxoSmithKline Inc. 2018).
To prevent IMD caused by serogroups A, C, Y, and W-135, infant and adolescent routine immunization programs are in place across Canada (Public Health Agency of Canada 2018). For protection against IMD caused by serogroup B, the National Advisory Committee on Immunization (NACI) recommendsFootnote 1 4CMenB vaccination for individuals at high risk of meningococcal disease and those who have had a close contact with a case, as well as to control outbreaks (National Advisory Committee on Immunization 2014). MnB vaccination is not currently included in Canada’s routine vaccination schedule but several provinces publicly fund the vaccine for recommended individuals at high risk of meningococcal disease. Adolescents and young adults are at highest risk of N. meningitidis carriage and transmission (Kaaijk et al. 2014), so routine MnB vaccination in this population could help reduce the burden of IMD in Canada.
The current model was developed to assess the cost-effectiveness of different strategies for adolescent MenB-FHbp vaccination in Canada.
Methods
Model description
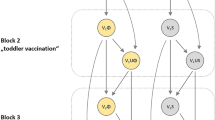
A population-based dynamic transmission model was developed to estimate the expected reduction of MnB IMD cases in the 30 years following introduction of routine age-targeted vaccination in the Canadian population. The model structure (Fig. 1) is similar to that published by Ortega-Sanchez et al. (2008). Meningococcal bacteria carriage is the source of infectious transmission and was the primary consideration in the model calculation. The population was stratified into 101 single-year age bands and individuals in each age band transitioned to the next age band in the following year. Each year, the model assumed that a proportion of individuals in each age group were serogroup B N. meningitidis carriers who had age-specific probabilities of developing IMD and transmitting the bacteria within their age group or across other age groups (Trotter et al. 2006; Trotter et al. 2002). To calculate meningococcal transmission, the population was stratified into 10 mutually exclusive age groups: 0 to 5 months, 6 to 12 months, 1 year, 2 to 4 years, 5 to 9 years, 10 to 14 years, 15 to 19 years, 20 to 24 years, 25 to 59 years, and ≥ 60 years. During each year in the model’s 30-year time horizon, a proportion of individuals in a targeted age group were vaccinated with MenB-FHbp. The vaccine was assumed to provide direct protection for vaccinated non-carriers against acquiring MnB or for existing carriers against developing IMD. Indirect protection of non-vaccinated individuals due to reduction of carriage prevalence and transmission was also assumed (Marshall et al. 2013; Read et al. 2014). The vaccine’s direct and indirect protection waned as the population aged. Individuals who developed IMD either recovered, with or without complications, or died.
Model disease inputs
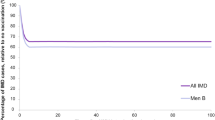
MnB incidence rates were derived from 2007 to 2011 Canadian national surveillance—the most recent data available at the time of model development (Fig. 2) (National Advisory Committee on Immunization 2014; National Advisory Committee on Immunization 2013). One scenario analysis assumed 20% higher incidence to address potential underestimation of reported IMD cases, and another used age-specific incidence rates in 2011 through 2016 surveillance data provided by the Public Health Agency of Canada reflecting reduced incidence of IMD in Canada (Public Health Agency of Canada n.d.). IMD-related short- or long-term complications included in the model were skin scarring, amputation, paralysis, seizures, hearing loss, neurologic sequelae, or renal failure. The probabilities of these complications were derived from a cohort study of the outcomes of IMD in adults and children in Canada between 2002 and 2014 (Table 1) (Sadarangani et al. 2015).
As there were no published age-specific MnB carriage data for Canada at the time of analysis, age- and serogroup-specific case-to-carrier ratios for a United Kingdom population (Trotter et al. 2006) were adapted and multiplied by Canadian MnB IMD incidence to derive an estimated baseline prevalence of pre-vaccine MnB carriage in Canada. These ratios represent the probability of disease given carriage and were also used to calculate the number of IMD cases per year and per age group based on the number of unprotected individuals and the estimated carriage prevalence after vaccine introduction.
The model population size was based on the annual Canadian population estimates tables for the year 2016 (Statistics Canada 2016b). The life expectancy at each age was obtained from the Canadian life expectancy table, which captures non-IMD deaths (Statistics Canada 2016a).
Meningococcal carriage transmission
The model’s dynamic population mixing matrix was based on a published MnC model where 98% of meningococcal transmission was contained within a 3-year age band of individuals 1 year older and younger than the affected carrier and the remaining 2% of the transmission was assumed to come from all other ages equally (Trotter et al. 2005).
In each model year, the number of nasopharyngeal meningococcal carriers in each age group was determined by 4 factors: (1) age-specific carriage prevalence in the prior year; (2) transmission of bacteria within and among age groups; (3) proportion of people vaccinated in each age group; and (4) vaccine efficacy against carriage acquisition. For example, in the 2 to 4 years age group, prevalence was calculated as follows: \( \mathrm{prevalence}\ {\left(\mathrm{year}\ n\right)}^{2-4\ \mathrm{years}}\sim {\upbeta}_1^{2-4}\times \mathrm{prevalence}{\left(\mathrm{year}\ n-1\right)}^{0-5\ \mathrm{months}}+{\upbeta}_2^{2-4}\times \mathrm{prevalence}{\left(\mathrm{year}\ n-1\right)}^{6-12\ \mathrm{months}}+\cdots +{\upbeta}_{10}^{2-4}\times \mathrm{prevalence}{\left(\mathrm{year}\ n-1\right)}^{\ge 60\ \mathrm{years}} \), where \( {\beta}_i^{2-4} \) represents the proportion of carriage in the 2 to 4 years age group originating from age group i and \( \sum {\beta}_i^{2-4}=1 \). Given the model’s assumption of protection against carriage acquisition, age-specific carriage prevalence decreases over time as a function of vaccine uptake, efficacy, and waning.
Vaccination scenarios
The model examined 3 immunization strategies using MenB-FHbp vaccine: (1) vaccination at age 14 years with 75% vaccine uptake; (2) vaccination at age 17 with 75% uptake; and (3) vaccination at age 17 with 30% uptake. The 75% uptake rate was estimated from immunization rates reported for school vaccination programs in Ontario for the 2012 to 2013 school year (Ontario Agency for Health Protection and Promotion (Public Health Ontario) 2014). The 30% uptake rate for 17-year-olds was an assumption for vaccination outside of a school setting for older adolescents and is consistent with the 34% uptake rate observed among 17- to 20-year-olds during the 2014 Saguenay Lac St-Jean immunization campaign (De Wals et al. 2017). All adolescents in the target groups were assumed to receive 2 doses of the MenB-FHbp vaccine. The possibility of partial vaccination (i.e., missed doses) was not considered in our model. For all scenarios, uptake was considered immediate upon vaccine introduction and constant over the 30-year time horizon.
Vaccine efficacy
A population-based study of infants in the UK estimated a 2-dose MnB vaccine effectiveness of 82.9% against MnB cases (Parikh et al. 2016), while a UK model estimated 95% MnB vaccine efficacy to prevent IMD among carriers (Christensen et al. 2016). A 75% to 100% seroresponse rate in adolescents was reported in clinical trials of a 2-dose regimen of the MenB-FHbp vaccine (Vesikari et al. 2016). Thus, we assumed a conservative estimate of 85% vaccine efficacy for adolescents (Table 2). We also assumed 26.6% efficacy against carriage acquisition based on published literature (Read et al. 2014). Although some studies have assumed a 10-year duration of efficacy for adolescent meningococcal vaccine protection (Christensen et al. 2010), we conservatively assumed a 5-year duration of vaccine efficacy against IMD, equal to what was observed with one of the conjugate MnACYW-135 vaccines and consistent with MenB-FHbp immunogenicity studies at 4-year follow-up (Cohn et al. 2017; Patton et al. 2017; Vesikari et al. 2017). Vaccine efficacy against IMD was assumed to decrease by 10% per year over the 5-year duration of protection. Vaccine efficacy against carriage acquisition was assumed to wane faster (20% per year) because higher antibody titers are likely necessary to protect against carriage acquisition. After the assumed duration of protection, both disease and carriage protection efficacy became 0% for the remainder of the 30-year horizon.
Costs and disutilities
Costs of model parameters were considered from the societal perspective and included medical costs associated with complicated or uncomplicated IMD treatment or death (e.g., hospital costs to treat IMD, costs of prosthesis after amputation) and costs of caregiver time or lost work (e.g., caregiver loss of work, lost future productivity due to death or neurologic sequelae). All costs were inflated to 2015 Canadian dollars and costs and utilities were discounted at 3% annually (Table 3).
Vaccination costs include cost of the vaccine and an administration cost of $10.10 per dose for a 2-dose schedule (De Wals et al. 2007). The vaccine price used in the analysis ($156.44 for the 2-dose series) is approximate as prices of publicly funded vaccines in Canada are confidentially negotiated with provinces through bulk procurement programs. There are currently no public contracts in place for the MenB-FHbp vaccine; hence, this value is not a negotiated and approved contract price.
A one-time quality-of-life loss, or disutility of 0.0317, is assumed to occur with each case. In addition, complicated IMD cases are assumed to accrue additional lifelong utility loss.
Sensitivity analyses
Univariate sensitivity analyses were conducted varying the vaccination costs, vaccine uptake, carriage and disease protection against disease, and epidemiology. Each of these parameters was increased and decreased by 20% to assess the relative impact of changes on the model outcomes. Sensitivity analyses were performed for each of the 3 vaccine strategies.
Results
Incremental cost-effectiveness ratios (ICERs) were calculated for each of the 3 vaccination strategies compared with no vaccination (Table 4). Without vaccination, 3974 MnB cases would be expected in Canada, resulting in 256 deaths and total disease-related costs of nearly $235 million over the 30-year time horizon. With 75% uptake, a routine MenB-FHbp vaccination program for 14-year-olds would yield an ICER of $975,954 per quality-adjusted life-year (QALY) and prevent 688 cases and 33 deaths, while vaccination of 17-year-olds with the same uptake would have an ICER of $684,654/QALY compared with no vaccination and could prevent an additional 345 cases and 22 deaths. If vaccination uptake among 17-year-olds were reduced to 30%, this would result in an ICER of $489,700/QALY and would prevent 575 cases and 30 deaths.
Results of univariate sensitivity analyses are similar for the 3 immunization strategies (Fig. 3). With an assumption of 75% vaccine uptake among 14- and 17-year-olds, the top 5 most sensitive variables in the model were disease incidence, vaccination costs, vaccine uptake rate, vaccine efficacy against carriage, and vaccine duration of protection against carriage or waning carriage protection, whereas vaccine efficacy against carriage was the third most sensitive variable in the 30% uptake at age 17 strategy, higher than vaccine uptake. Of note, increases in vaccine uptake result in higher ICERs due to the impact of indirect immunity. That is, additional vaccination in unvaccinated individuals incurs the full costs of vaccination but accrues proportionally smaller additional disease benefits because those who were unvaccinated already had some benefit via indirect protection. Results of scenarios analyses are presented in Table 5.
Univariate sensitivity analyses of key parameters for vaccination. Strategy 1 (age 14 with 75% uptake), strategy 2 (age 17 with 75% uptake), and strategy 3 (age 17 with 30% uptake). ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-year. The centre of each plot corresponds to the base case ICER obtained for each of the 3 scenarios (Table 4). The horizontal bars represent the ICER with each parameter 20% higher (white bars) or 20% lower (black bars) than the base case
Discussion
Our model demonstrates that routine adolescent MenB-FHbp vaccination could have a substantial public health impact in Canada, although it did not reach commonly accepted cost-effectiveness thresholds (Walker et al. 2010). Even at less than half the vaccine uptake (30% vs 75%), vaccinating older adolescents (age 17 vs age 14) against MnB is associated with improved health outcomes and lower costs per QALY. This finding is due to age-related differences in disease incidence and carriage prevalence. At age 14, MnB protection begins during a period of relatively low incidence (0.23 cases/100,000) and decreases each subsequent year just as incidence increases to 0.73 cases per 100,000 for ages 15 through 19. In contrast, vaccination at age 17 is assumed to provide optimal protection during a period in which the incidence (0.43 cases/100,000 for ages 20 through 24) and expected carriage are higher than at age 14. For this reason, immunization at 17 years old may be a better option to provide optimal protection as well as cost-effectiveness, though achieving high immunization rates in that age group would likely constitute a challenge.
The relatively low incidence of MnB in Canada (ranging from 0.2 to 0.3 cases per 100,000 population between 2011 and 2015 (Public Health Agency of Canada 2017)) may make routine vaccination less acceptable from a cost-effectiveness perspective, relative to more common but less severe diseases. However, decision-makers should also consider and prioritize preventing the significant morbidity or mortality associated with diseases such as MnB. Black (2013) illustrated how the use of cost-effectiveness analyses alone could lead to biased favourable decisions toward vaccines that provide economic benefit rather than those that reduce severe morbidity and mortality, such as vaccines against IMD.
The most significant limitations to the current model are the lack of data to estimate meningococcal transmission and the uncertainty of the vaccine efficacy against carriage. There is very limited information on the prevalence of N. meningitidis carriage in general, so carriage estimates for our model were calculated using MnB and MnC studies in a UK population. The longitudinal study of 2010 to 2013 MnB bacteria throat carriage prevalence among 13- to 25-year-olds in Quebec (Gilca et al. 2018) was not available at the time of our analysis. Nevertheless, our estimates of carriage prevalence in the 15 to 19 years age group were similar to those reported by Gilca et al. for 9th and 11th graders. We adapted the UK-based MnC population mixing matrix from Trotter et al. (2005) for estimating MnB-specific transmission data. Similar to our findings, results of other published health economic evaluations of MnB vaccines were shown to be sensitive to assumptions surrounding disease incidence, mortality, and vaccine protection against carriage (Black 2013; Drummond et al. 2007; Getsios et al. 2004; Kauf 2010). Hence, small changes in the model assumptions regarding disease and carriage could result in potentially different conclusions. However, we believe the current results are conservative estimates of the benefits of adolescent vaccinations as our assumptions regarding vaccine uptake, indirect costs, and effectiveness against carriage were lower compared with those reported in other published 4CMenB economic analyses (Pan-Canadian Public Health Network et al. 2014).
With regard to discounting, the UK’s National Institute for Health and Care Excellence (NICE) and the Canadian Agency for Drugs and Technologies in Health (CADTH) both recommend the use of a 1.5% discount rate for costs and benefits (Canadian Agency for Drugs and Technologies in Health 2017), as higher discounting rates could undervalue the benefit of preventive and public health projects that have large costs up front but accrue benefits over decades (Rawlins et al. 2010). Using a 1.5% discount rate in our model yielded 35% lower ICER ratios compared with the 3% used for the base case.
The results of this study are in line with previous cost-effectiveness model studies conducted mainly for infant MnB vaccination in Canada and Europe, which also resulted in ICERs beyond current acceptability thresholds. Tu et al. (2014) conducted an economic evaluation of infant MnB vaccination in Ontario using higher efficacy assumptions (90% vaccine effectiveness, 66% strain coverage, 97% vaccine coverage) than our model, and concluded that the program was unlikely to be considered economically attractive in Canada. Although vaccinating a cohort of 150,000 infants could be expected to prevent 4.6 MnB IMD cases and 0.5 related deaths over the cohort’s lifetime, cost savings from prevented cases could not offset the vaccination program cost, resulting in an ICER of CAD $4.76 million per QALY gained.
Traditional health economic evaluations do not appear to capture the full impact of a MnB vaccination program. Analyses in The Netherlands (Pouwels et al. 2013), Italy (Gasparini et al. 2016), England (Christensen et al. 2013), and France (Lecocq et al. 2016) all found an infant immunization program not to be cost-effective. However, when the authors of the English study included more “favourable” assumptions, including quality-of-life losses for family and network members, 1.5% discounting, and the value of litigation costs, results showed that a routine infant vaccination could be cost-effective with a lower vaccine price (Christensen et al. 2016). In our analysis, in order to obtain a cost per QALY of $135,000 (considered the highest limit of cost-effectiveness according to the WHO guidelines (Walker et al. 2010)), the disease incidence would have to be 4.65 times higher (even more if using the most recent data (Public Health Agency of Canada 2017)) or a vaccine price of $11 per dose.
Conclusion
MnB disease is rare but potentially devastating and life-threatening. A significant number of cases occur in adolescents and young adults, who are also at higher risk of carrying N. meningitidis and transmitting the disease. Although the implementation of routine adolescent MenB-FHbp vaccination could have a substantial public health impact in preventing IMD cases and deaths, given the current low incidence of MnB disease in Canada, our results show that such a program would not be cost-effective. As cost-effectiveness plays a significant role in the current evaluation framework of new vaccines in Canada, the potential for recommendation and funding of an immunization program with an ICER beyond acceptable thresholds is low. However, IMD incidence has been historically unpredictable; other criteria such as prevention of outbreaks, peace of mind for parents and society, ethical considerations, and the potential positive impact of vaccination on antimicrobial resistance should be considered in the decision-making.
Notes
At the time of manuscript writing, NACI had not yet issued its recommendation for MenB-FHbp.
References
Bettinger, J. A., Scheifele, D. W., Le Saux, N., Halperin, S. A., Vaudry, W., Tsang, R., et al. (2013). The disease burden of invasive meningococcal serogroup B disease in Canada. The Pediatric Infectious Disease Journal, 32(1), e20–e25. https://doi.org/10.1097/INF.0b013e3182706b89.
Bijlard, E., Kouwenberg, C. A., Timman, R., Hovius, S. E., Busschbach, J. J., & Mureau, M. A. (2017). Burden of keloid disease: a cross-sectional health-related quality of life assessment. Acta Dermato-Venereologica, 97(2), 225–229. https://doi.org/10.2340/00015555-2498.
Black, S. (2013). The role of health economic analyses in vaccine decision making. Vaccine, 31(51), 6046–6049. https://doi.org/10.1016/j.vaccine.2013.08.008.
Canadian Agency for Drugs and Technologies in Health (2017). Guidelines for the economic evaluation of health technologies: Canada. Ottawa: https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf.
Chen, J. M., Amoodi, H., & Mittmann, N. (2014). Cost-utility analysis of bilateral cochlear implantation in adults: a health economic assessment from the perspective of a publicly funded program. Laryngoscope, 124(6), 1452–1458. https://doi.org/10.1002/lary.24537.
Christensen, H., Hickman, M., Edmunds, W. J., & Trotter, C. L. (2013). Introducing vaccination against serogroup B meningococcal disease: an economic and mathematical modelling study of potential impact. Vaccine, 31(23), 2638–2646. https://doi.org/10.1016/j.vaccine.2013.03.034.
Christensen, H., Irving, T., Koch, J., Trotter, C. L., Ultsch, B., Weidemann, F., et al. (2016). Epidemiological impact and cost-effectiveness of universal vaccination with Bexsero® to reduce meningococcal group B disease in Germany. Vaccine, 34(29), 3412–3419. https://doi.org/10.1016/j.vaccine.2016.04.004.
Christensen, H., May, M., Bowen, L., Hickman, M., & Trotter, C. L. (2010). Meningococcal carriage by age: a systematic review and meta-analysis. The Lancet Infectious Diseases, 10(12), 853–861. https://doi.org/10.1016/S1473-3099(10)70251-6.
Cohn, A. C., MacNeil, J. R., Harrison, L. H., Lynfield, R., Reingold, A., Schaffner, W., et al. (2017). Effectiveness and duration of protection of one dose of a meningococcal conjugate vaccine. Pediatrics, 139(2). https://doi.org/10.1542/peds.2016-2193.
Crum-Cianflone, N., & Sullivan, E. (2016). Meningococcal vaccinations. Infectious Diseases and Therapy, 5(2), 89–112. https://doi.org/10.1007/s40121-016-0107-0.
De Wals, P., Coudeville, L., Trottier, P., Chevat, C., Erickson, L. J., & Nguyen, V. H. (2007). Vaccinating adolescents against meningococcal disease in Canada: a cost-effectiveness analysis. Vaccine, 25(29), 5433–5440. https://doi.org/10.1016/j.vaccine.2007.04.071.
De Wals, P., Deceuninck, G., Lefebvre, B., Tsang, R., Law, D., De Serres, G., et al. (2017). Impact of an immunization campaign to control an increased incidence of serogroup B meningococcal disease in one region of Quebec, Canada. Clinical Infectious Diseases, 64(9), 1263–1267. https://doi.org/10.1093/cid/cix154.
Drummond, M., Chevat, C., & Lothgren, M. (2007). Do we fully understand the economic value of vaccines? Vaccine, 25(32), 5945–5957. https://doi.org/10.1016/j.vaccine.2007.04.070.
Gasparini, R., Tregnaghi, M., Keshavan, P., Ypma, E., Han, L., & Smolenov, I. (2016). Safety and immunogenicity of a quadrivalent meningococcal conjugate vaccine and commonly administered vaccines after coadministration. The Pediatric Infectious Disease Journal, 35(1), 81–93. https://doi.org/10.1097/inf.0000000000000930.
Getsios, D., Caro, I., El-Hadi, W., & Caro, J. J. (2004). Assessing the economics of vaccination for Neisseria meningitidis in industrialized nations: a review and recommendations for further research. International Journal of Technology Assessment in Health Care, 20(3), 280–288.
Gilca, R., De Wals, P., Nolan, S. M., Kitchin, N., Eiden, J. J., Jiang, Q., et al. (2018). A longitudinal epidemiology study of meningococcal carriage in students 13 to 25 years old in Quebec. mSphere, 3(6), e00427–e00418. https://doi.org/10.1128/mSphere.00427-18.
Ginsberg, G. M., Block, C., & Stein-Zamir, C. (2016). Cost-utility analysis of a nationwide vaccination programme against serogroup B meningococcal disease in Israel. International Journal of Public Health, 61(6), 683–692. https://doi.org/10.1007/s00038-016-0821-0.
GlaxoSmithKline Inc. (2018). Product monograph: Bexsero multicomponent meningococcal B vaccine (recombinant, adsorbed). http://ca.gsk.com/media/1212390/bexsero.pdf. Accessed 7 Nov 2018.
Gu, W., & Wong, A. (2010). Estimates of human capital in Canada: the lifetime income approach. Economic Analysis Research Paper Series, No. 62. Ottawa: Statistics Canada. http://www.statcan.gc.ca/pub/11f0027m/11f0027m2010062-eng.pdf.
Kaaijk, P., van der Ende, A., & Luytjes, W. (2014). Routine vaccination against MenB: considerations for implementation. Human Vaccines & Immunotherapeutics, 10(2), 310–316. https://doi.org/10.4161/hv.26816.
Kauf, T. L. (2010). Methodological concerns with economic evaluations of meningococcal vaccines. Pharmacoeconomics, 28(6), 449–461. https://doi.org/10.2165/11535280-000000000-00000.
Langley, J. M., MacDougall, D. M., Halperin, B. A., Swain, A., Halperin, S. A., Top, K. A., et al. (2016). Rapid surveillance for health events following a mass meningococcal B vaccine program in a university setting: a Canadian Immunization Research Network study. Vaccine, 34(34), 4046–4049. https://doi.org/10.1016/j.vaccine.2016.06.025.
Lecocq, H., Parent du Chatelet, I., Taha, M. K., Levy-Bruhl, D., & Dervaux, B. (2016). Epidemiological impact and cost-effectiveness of introducing vaccination against serogroup B meningococcal disease in France. Vaccine, 34(19), 2240–2250. https://doi.org/10.1016/j.vaccine.2016.03.020.
Li, Y. A., Tsang, R., Desai, S., & Deehan, H. (2014). Enhanced surveillance of invasive meningococcal disease in Canada, 2006-2011. Canada Communicable Disease Report, 40-9. https://doi.org/10.14745/ccdr.v40i09a01
Marshall, H. S., Richmond, P. C., Nissen, M. D., Wouters, A., Baber, J., Jiang, Q., et al. (2013). A phase 2 open-label safety and immunogenicity study of a meningococcal B bivalent rLP2086 vaccine in healthy adults. Vaccine, 31(12), 1569–1575. https://doi.org/10.1016/j.vaccine.2013.01.021.
Murray, C. J. L., & Lopez, A. D. (1996). The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries, and risk factors in 1990 and projected to 2020 (Global burden of disease and injury series, Vol. 1). Cambridge, MA: Published by the Harvard School of Public Health on behalf of the World Health Organization and the World Bank; Distributed by Harvard University Press.
National Advisory Committee on Immunization (2013). Update on the use of quadrivalent conjugate meningococcal vaccines. An Advisory Committee Statement (ACS). Canada Communicable Disease Report, 39(ACS-1). https://doi.org/10.14745/ccdr.v39i00a01
National Advisory Committee on Immunization (2014). Advice for the use of the multicomponent meningococcal serogroup B (4CMenB) vaccine. An Advisory Committee Statement (ACS). http://publications.gc.ca/collections/collection_2014/aspc-phac/HP40-104-2014-eng.pdf. Accessed 8 Jan 2018.
Nova Scotia Department of Health and Wellness (2015). Communicable diseases - meningococcal meningitis. https://novascotia.ca/dhw/CDPC/meningitis.asp. Accessed 21 Sept 2018.
Ontario Agency for Health Protection and Promotion (Public Health Ontario) (2014). Immunization coverage report for school pupils: 2012-13 school year. Toronto, ON: Queen’s Printer for Ontario. https://www.publichealthontario.ca/en/eRepository/Immunization_coverage_report_2012-13.pdf.
Ontario Case Costing Initiative (OCCI). OCCI Costing Analysis Tool. (2016). Available from: https://hsimi.ca/occp/occpreports/. Accessed 10/25/2018.
Ontario Ministry of Health and Long-Term Care (2015). Meningococcal Fact Sheet for Parents and Students. http://www.health.gov.on.ca/en/public/publications/immune/meningococcal_ps.aspx. Accessed 14 Nov 2016.
Oostenbrink, R., Moll, H. A., & Essink-Bot, M. L. (2002). The EQ-5D and the Health Utilities Index for permanent sequelae after meningitis: a head-to-head comparison. Journal of Clinical Epidemiology, 55(8), 791–799.
Ortega-Sanchez, I. R., Meltzer, M. I., Shepard, C., Zell, E., Messonnier, M. L., Bilukha, O., et al. (2008). Economics of an adolescent meningococcal conjugate vaccination catch-up campaign in the United States. Clinical Infectious Diseases, 46(1), 1–13.
Pan-Canadian Public Health Network, Partners in Public Health, & Meningococcal B Pilot Project Task Group (2014). The recommended use of the multicomponent meningococcal B (4CMenB) vaccine in Canada. http://www.phac-aspc.gc.ca/naci-ccni/mening-4cmenb-exec-resum-eng.php. Accessed 16 Aug 2016.
Parikh, S. R., Andrews, N. J., Beebeejaun, K., Campbell, H., Ribeiro, S., Ward, C., et al. (2016). Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet, 388(10061), 2775–2782. https://doi.org/10.1016/s0140-6736(16)31921-3.
Patton, M. E., Stephens, D., Moore, K., & MacNeil, J. R. (2017). Updated recommendations for use of MenB-FHbp serogroup B meningococcal vaccine - Advisory Committee on Immunization Practices, 2016. MMWR. Morbidity and Mortality Weekly Report, 66(19), 509–513. https://doi.org/10.15585/mmwr.mm6619a6.
Pfizer Canada Inc. (2018). Trumenba [product monograph including patient medication information]. https://www.pfizer.ca/sites/g/files/g10050796/f/201808/Trumenba_PM_214857_24-Jul-2018_E.pdf.
Pouwels, K. B., Hak, E., van der Ende, A., Christensen, H., van den Dobbelsteen, G. P., & Postma, M. J. (2013). Cost-effectiveness of vaccination against meningococcal B among Dutch infants: crucial impact of changes in incidence. Human Vaccines & Immunotherapeutics, 9(5), 1129–1138. https://doi.org/10.4161/hv.23888.
Public Health Agency of Canada (2015). Invasive Meningococcal Disease. Available from: http://www.phac-aspc.gc.ca/im/vpd-mev/meningococcal/professionals-professionnels-eng.php.
Public Health Agency of Canada (2017). Vaccine preventable disease: surveillance report to December 31, 2015. https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/vaccine-preventable-disease-surveillance-report-december-31-2015/vaccine-preventable-disease-eng.pdf.
Public Health Agency of Canada (2018). Canada’s provincial and territorial routine (and catch-up) vaccination routine schedule programs for infants and children. https://www.canada.ca/en/public-health/services/provincial-territorial-immunization-information/provincial-territorial-routine-vaccination-programs-infants-children.html. Accessed 20 Sept 2018.
Public Health Agency of Canada (n.d.) National Enhanced Invasive Meningococcal Disease Surveillance System. Accessed December 3, 2018.
Rancourt, C., Gregoire, J. P., Simons, W., & Dostie, A. (2003). Cost-benefit model comparing two alternative immunisation programmes against serogroup C meningococcal disease: for Quebec residents aged 2 months to 20 years. Pharmacoeconomics, 21(6), 429–442.
Rawlins, M., Barnett, D., & Stevens, A. (2010). Pharmacoeconomics: NICE’s approach to decision-making. British Journal of Clinical Pharmacology, 70(3), 346–349. https://doi.org/10.1111/j.1365-2125.2009.03589.x.
Read, R. C., Baxter, D., Chadwick, D. R., Faust, S. N., Finn, A., Gordon, S. B., et al. (2014). Effect of a quadrivalent meningococcal ACWY glycoconjugate or a serogroup B meningococcal vaccine on meningococcal carriage: an observer-blind, phase 3 randomised clinical trial. Lancet, 384(9960), 2123–2131. https://doi.org/10.1016/S0140-6736(14)60842-4.
Sadarangani, M., Scheifele, D. W., Halperin, S. A., Vaudry, W., Le Saux, N., Tsang, R., et al. (2015). Outcomes of invasive meningococcal disease in adults and children in Canada between 2002 and 2011: a prospective cohort study. Clinical Infectious Diseases, 60(8), e27–e35. https://doi.org/10.1093/cid/civ028.
Shepard, C. W., Ortega-Sanchez, I. R., Scott, R. D., & Rosenstein, N. E. (2005). Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics, 115(5), 1220–1232.
Statistics Canada (2016a). Table 13-10-0114-01 Life expectancy and other elements of the life table, Canada, all provinces except Prince Edward Island. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310011401. Accessed 19 July 2016.
Statistics Canada (2016b). Table 17-10-0005-01 (formerly CANSIM 051-0001): population estimates on July 1st, by age and sex. https://www150.statcan.gc.ca/t1/tbl1/en/cv.action?pid=1710000501. Accessed 14 July 2016.
Trotter, C. L., Gay, N. J., & Edmunds, W. J. (2005). Dynamic models of meningococcal carriage, disease, and the impact of serogroup C conjugate vaccination. American Journal of Epidemiology, 162(1), 89–100.
Trotter, C. L., Gay, N. J., & Edmunds, W. J. (2006). The natural history of meningococcal carriage and disease. Epidemiology and Infection, 134(3), 556–566.
Trotter, C. L., Ramsay, M. E., & Kaczmarski, E. B. (2002). Meningococcal serogroup C conjugate vaccination in England and Wales: coverage and initial impact of the campaign. Communicable Disease and Public Health, 5(3), 220–225.
Tu, H. A., Deeks, S. L., Morris, S. K., Strifler, L., Crowcroft, N., Jamieson, F. B., et al. (2014). Economic evaluation of meningococcal serogroup B childhood vaccination in Ontario, Canada. Vaccine, 32(42), 5436–5446. https://doi.org/10.1016/j.vaccine.2014.07.096.
Vesikari, T., Østergaard, L., Diez-Domingo, J., Wysocki, J., Flodmark, C.-E., Beeslaar, J., et al. (2016). Meningococcal serogroup B bivalent rLP2086 vaccine elicits broad and robust serum bactericidal responses in healthy adolescents. J Pediatric Infect Dis Soc, 5(2), 152–160. https://doi.org/10.1093/jpids/piv039.
Vesikari, T., Ostergaard, L., Beeslaar, J., Eiden, J. J., & Jansen, K. U. Persistence and 4-year boosting of the bactericidal response elicited by 2- and 3-dose schedules of MenB-FHBP. 35th Annual Meeting of the European Society for Pediatric Infectious Diseases, Madrid, Spain, 2017.
Walker, D. G., Hutubessy, R., & Beutels, P. (2010). WHO Guide for standardisation of economic evaluations of immunization programmes. Vaccine, 28(11), 2356–2359. https://doi.org/10.1016/j.vaccine.2009.06.035.
World Health Organization (2018). Meningococcal meningitis. Fact sheet N°141 (v1.0). https://www.who.int/news-room/fact-sheets/detail/meningococcal-meningitis. Accessed 20 Dec 2018.
Acknowledgements
The authors thank Catherine Mirvis of Pharmerit International for her assistance in writing the manuscript.
Funding
This study was sponsored by Pfizer Inc., the manufacturer of MenB-FHbp.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
At the time of analysis, SJ Snedecor and N Cornelio are/were employees of Pharmerit International, which received financial support from Pfizer Inc. to conduct this study and for manuscript development. M-C Breton, L Huang, and F Fanton-Aita are employees of Pfizer.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Breton, MC., Huang, L., Snedecor, S.J. et al. Cost-effectiveness of alternative strategies for vaccination of adolescents against serogroup B IMD with the MenB-FHbp vaccine in Canada. Can J Public Health 111, 182–192 (2020). https://doi.org/10.17269/s41997-019-00275-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.17269/s41997-019-00275-4
Keywords
- Cost-effectiveness analysis
- Meningococcal vaccine
- Meningococcal disease
- Canada
- Adolescents
- Transmission dynamic model
- Economic model