-
PDF
- Split View
-
Views
-
Cite
Cite
Milica Požgayová, Radka Piálková, Marcel Honza, Petr Procházka, Sex-specific nestling growth in an obligate brood parasite: Common Cuckoo males grow larger than females, The Auk, Volume 135, Issue 4, 1 October 2018, Pages 1033–1042, https://doi.org/10.1642/AUK-18-26.1
Close - Share Icon Share
Abstract
Growth is a key life history trait that is closely related to individual fitness. In altricial birds, growth is restricted to a relatively short period, and depends primarily on the amount or quality of food and hence on parental care. Obligate brood parasites do not care for their own offspring but impose this burden on other species (hosts). As many brood parasites exploit various host species, their progeny are expected to receive different levels of parental care. Parasite growth has thus often been explored in the context of host parenting abilities and only rarely with respect to its sex specificity. Here, we fill this gap in knowledge and explore sex differences in the growth of Common Cuckoo (Cuculus canorus) nestlings reared by 2 warbler hosts in the genus Acrocephalus. As adult Common Cuckoo males are 5–16% heavier than females, we assumed that nestlings would also differ in size and thus in growth performance. To test this assumption, we used a nonlinear mixed effects modeling approach to fit an overall logistic curve across all nestling masses and ages. We chose the logistic growth model over its alternatives because it is one of the most used models for birds and it is suitable for the growth of Common Cuckoo nestlings. We found that both sexes exhibited similar mass after hatching and grew at a similar rate. Nevertheless, males reached ∼10% higher asymptotic mass than females, while fledging at a similar age as females. These findings imply that male Common Cuckoo nestlings may have higher needs than female nestlings; however, this still awaits proper testing.
Resumen
El crecimiento es un rasgo clave de la historia de vida relacionado íntimamente con la adecuación biológica del individuo. En las aves altriciales, está restringido a un período relativamente corto y depende primariamente de la cantidad o calidad del alimento y por ende del cuidado parental. Los parásitos obligados de nidada no se preocupan por su propia descendencia sino que le imponen esta carga a otras especies (hospederos). Debido a que muchos parásitos de nidada explotan varias especies hospederas, se espera que su progenie reciba distintos niveles de cuidado parental. El crecimiento de los parásitos ha sido por ende usualmente estudiado en el contexto de las habilidades de los hospederos y solo de manera muy limitada con respecto a la especificidad del sexo. Aquí, completamos esta falta de conocimiento y exploramos las diferencias de sexo en el crecimiento de los volantones de Cuculus canorus criados por dos hospederos del género Acrocephalus. Debido a que los machos de C. canorus son 5–16% más pesados que las hembras, asumimos que los volantones también se diferenciarían en el tamaño y por ende en el desempeño de crecimiento. Para evaluar este supuesto, usamos un enfoque de modelado de efectos mixtos no lineales para ajustar una curva logística general a todos los pesos y las edades de los volantones. Elegimos el modelo de crecimiento logístico sobre otras alternativas debido a que es uno de los modelos más usados en aves y es adecuado para el crecimiento de los volantones de C. canorus. Encontramos que ambos sexos exhibieron un peso similar luego de la eclosión y que crecieron a una tasa similar. Sin embargo, los machos alcanzaron una asíntota de peso un ∼10% más alta que las hembras, mientras que emplumaron a la misma edad que las hembras. Estos resultados implican que los volantones macho de C. canorus pueden tener mayor necesidad que los volantones hembra; sin embargo, esto aún debe ser evaluado adecuadamente.
Palabras clave: Acrocephalus arundinaceus, A. scirpaceus, Cuculus canorus, desarrollo, dimorfismo sexual de tamaño, ontogenia
Introduction
Ontogeny is an important stage of life, strongly associated with individual fitness (reviewed by Gebhardt-Henrich and Richner 1998, Lindström 1999, Metcalfe and Monaghan 2001). Growth, as an important ontogenetic process, is relatively well studied in birds, because birds are quite common, exhibit relatively short developmental periods, and have evolved various phenotypes along the altricial–precocial continuum (Starck and Ricklefs 1998a). At the individual level, growth may be affected by offspring genotype, physiology, rearing conditions (weather, food, siblings), and maternally induced effects (egg characteristics, timing of incubation), as well as offspring and parental behavior (for review see, e.g., Gebhardt-Henrich and Richner 1998, Ricklefs et al. 1998, Mainwaring and Hartley 2012).
Offspring sex can significantly influence growth and survival, particularly in species with adults that are sexually dimorphic in size (Ricklefs 1968, Richner 1991, Teather and Weatherhead 1994). Sex-specific differences in size may then influence the nutritional requirements of sons and daughters (see Magrath et al. 2007 and references therein), increasing the vulnerability of the larger sex to starvation under unfavorable conditions (Daunt et al. 2001, Benito and González-Solís 2007, Loonstra et al. 2018, but see Råberg et al. 2005, Rowland et al. 2007) or, contrarily, increasing its success in sibling competition (Teather 1992, Nicolaus et al. 2009). With respect to rearing conditions, the quantity and quality of delivered food and parental care as a whole play decisive roles in determining offspring growth patterns (Martin 1987, Starck and Ricklefs 1998b, Scheuerlein and Gwinner 2006).
Not all parents, however, are willing to undertake the onerous burden of parental care. Obligate brood parasites exhibit an alternative reproductive strategy wherein they lay eggs in the nests of other species (hosts), which subsequently care for their offspring (Rothstein and Robinson 1998, Davies 2000). Apart from strict host specialists, the majority of avian brood parasitic species exploit various numbers of host species that differ in many characteristics, most typically in body size. As this trait is often related to the quality and intensity of parental care (Sæther 1994), the growth of brood parasites has often been explored in the context of host size differences (Wiley 1986, Weatherhead 1989, Kleven et al. 1999, Kilpatrick 2002, Grim 2006, Grim and Samaš 2016, Li et al. 2016, but see Remeš 2010).
Among other traits affecting the growth and survival of parasitic nestlings (see, e.g., Lorenzana and Sealy 1999, Hauber 2003, Hauber and Moskát 2008), the sex of the nestling has received relatively little attention. However, there are studies that have explored this issue in parasitic cowbirds (Weatherhead 1989, Tonra et al. 2008, De Mársico et al. 2010, Tuero et al. 2013). Although Weatherhead (1989) found that the sexual size dimorphism seen in adult Brown-headed Cowbirds (Molothrus ater) was not expressed in their nestlings, later studies on this and other parasitic cowbird species showed that the sex of the parasitic nestling was the most important predictor of growth rate and mass before fledging, with males growing faster and fledging with higher mass than females (Tonra et al. 2008, De Mársico et al. 2010, Tuero et al. 2013). To our knowledge, no similar study on sex-specific nestling growth has ever been conducted on any other brood parasitic species. This is quite surprising, as the parasitic cowbirds represent less virulent agents (as they typically grow up alongside host nestlings) than some parasitic cuckoos or honeyguides that evict or kill their host siblings soon after hatching (Davies 2000).
To address this gap in our knowledge, we explored sex-specific nestling growth and fledging age in an evictor brood parasite, the Common Cuckoo (Cuculus canorus; hereafter, ‘cuckoo'). The cuckoo is sexually dimorphic in size, with adult males 5–16% heavier than adult females (Seel 1977, Glutz von Blotzheim and Bauer 1980, P. Procházka personal observation). Assuming that nestling mass before fledging is a good indicator of mass and thus individual quality in adulthood (e.g., Magrath 1991, Both et al. 1999, Cleasby et al. 2010), we predicted that male nestlings would grow heavier than female nestlings. Based on the general pattern of growth in sexually dimorphic birds, in which the larger sex attains its fledging mass at the same time as the smaller sex (Richner 1991), we further expected cuckoo males to fledge at a similar age as females.
Methods
Data Collection
This study was conducted from the beginning of May through mid-July of 2012–2017 at fishponds near Hodonín (48.85°N, 17.12°E) and Mutěnice (48.90°N, 17.03°E) in the Czech Republic. Here, we searched for host (Great Reed Warbler [Acrocephalus arundinaceus] and Eurasian Reed Warbler [Acrocephalus scirpaceus]) nests in littoral vegetation and checked them daily during egg laying to detect cuckoo eggs. As the expected hatching date approached (after 11–12 days of incubation; P. Procházka personal observation), the nests with accepted cuckoo eggs were again visited daily to determine the hatching day (i.e. the first day of age). Starting typically on this day, we weighed each nestling at 1- to 3-day (seldom longer) intervals using a digital pocket scale (model YA302, Ohaus Corporation, Parsippany, New Jersey, USA; accuracy = 0.1 g) until the age of 18 days. We chose the maximum age at weighing for ethical reasons to prevent premature fledging, which may negatively affect nestling survival. Because of logistic and natural reasons (i.e. predation, starvation, drowning, etc.), not all nestlings could be weighed until this age. Each nestling was measured 9 ± 2 times (mean ± SD; range: 3–15).
After termination of measurements, we continued with nest checks at 1- to 3-day intervals to assess cuckoo fledging date. Fledging date was determined as the date when the fledgling was first detected outside the nest and alive or was based on host behavior (feeding, warning calls). When the interval between the 2 nest checks was longer than one day, the fledging date was set as the middle of the interval between the last negative (nestling still in the nest) and the first positive (nestling already fledged) checks.
When nestlings were at least 6 days old, a small amount of blood (5–25 μL) was collected by tarsal venipuncture and stored in 100 μL of 96% ethanol. Nestling sex was later determined in the laboratory by amplifying a part of the W-linked chromodomain helicase DNA binding gene CHD-W (unique to females) and a part of its homologue, the CHD-Z gene, linked to the Z chromosome (common to both sexes; Griffiths et al. 1998). After DNA extraction, the avian sex primers P2 and P8 (Griffiths et al. 1998) were used in 10 μL PCR reactions (for details of PCR conditions, see Abraham et al. 2015). The PCR products were separated by electrophoresis for 45–60 min at 7–10 V cm−1 using 3% agarose gels stained with Syber stain (Invitrogen, Carlsbad, California, USA). Heterogametic females were characterized by a 2-band profile (∼350 and ∼400 bp), whereas homogametic males had only a single band (∼350 bp). DNA samples from adult males and females captured at the same study site were used as controls.
Statistical Analyses
All statistical analyses were conducted in R 3.3.1 (R Core Team 2016). Based on the growth pattern of cuckoo nestlings (Figure 1), we applied a 3-parameter logistic function (Starck and Ricklefs 1998b):
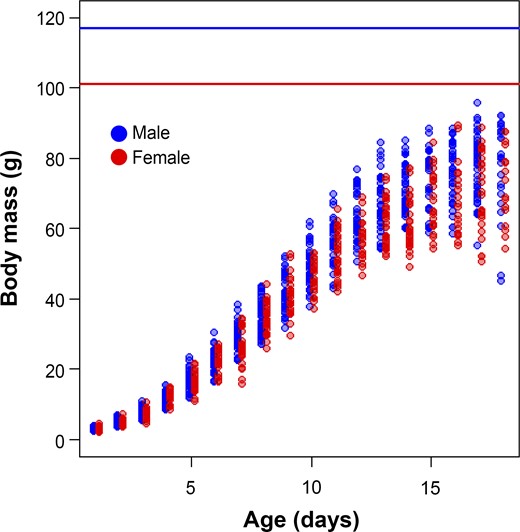
Sex-specific growth patterns of Common Cuckoo nestlings reared by 2 warbler hosts in the genus Acrocephalus (points represent raw data), and average mass of Common Cuckoo adults (shown by horizontal lines).
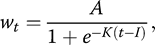
where t is nestling age (days; hatching day = day 1); wt is mass (g) at age t; A is the mass (g) asymptote (or upper plateau) of the growth curve, where mass reaches its highest value; K is the growth rate constant (day−1); and I is the age (days) at the inflection point (i.e. point of maximum growth) of the growth curve, where
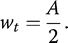
Following Sofaer et al. (2013), we did not fit separate logistic curves for individual nestlings, but instead fitted an overall curve across all nestling masses and ages. For this purpose, we used nonlinear mixed effects models with maximum likelihood in R package nlme (Pinheiro et al. 2016), where the mass of each nestling at a given age was treated as the dependent variable, and nestling sex, host species, and nestling hatching date were included as fixed effects (see below). To control for repeated measurements of the same individuals and to estimate the amount of interindividual variation, we included nestling identity as a random intercept (Sofaer et al. 2013, Aldredge 2016, Hildebrandt and Schaub 2018).
To analyze the growth data, we first had to determine the most appropriate structure for the random effects (Table 1). Therefore, we fit a set of models with the fixed effects in the form of the 3-parameter logistic function (see above) and with random effects for one or more of the growth parameters (A, K, and I), allowing the estimates of these parameters to vary among nestlings (see also Sofaer et al. 2013, Aldredge 2016). In these models, a growth parameter that did not include the random effect was characterized only by a fixed effect, whereas a growth parameter that included the random effect was characterized by both the fixed and random effects.
Results of model selection based on different structure of the random effects for the growth of Common Cuckoo nestlings reared by 2 warbler hosts in the genus Acrocephalus. In all models, the fixed effects took the form of a 3-parameter logistic function (fitted across all nestling masses and ages) without covariates. A = asymptotic mass (g), K = growth rate constant (day−1), I = inflection point of the growth curve (days), corr() = correlation between random effects at the nestling level. For further analyses, we selected the model highlighted in bold font, as the most-supported model was overparameterized. ΔAIC is the difference from the top model in Akaike's information criterion, log(L) is the log-likelihood of the model, and wi is the Akaike model weight.
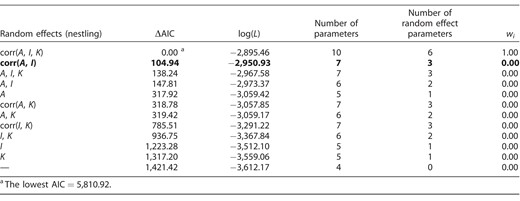
Results of model selection based on different structure of the random effects for the growth of Common Cuckoo nestlings reared by 2 warbler hosts in the genus Acrocephalus. In all models, the fixed effects took the form of a 3-parameter logistic function (fitted across all nestling masses and ages) without covariates. A = asymptotic mass (g), K = growth rate constant (day−1), I = inflection point of the growth curve (days), corr() = correlation between random effects at the nestling level. For further analyses, we selected the model highlighted in bold font, as the most-supported model was overparameterized. ΔAIC is the difference from the top model in Akaike's information criterion, log(L) is the log-likelihood of the model, and wi is the Akaike model weight.

Next, we extended the fixed effects by adding nestling sex (female, male), host species (Great Reed Warbler, Eurasian Reed Warbler), and nestling hatching date (centered, as it differed between the 2 host species) as covariates (Table 2). As these factors could affect the growth parameters independently, we decided to always retain the 3-parameter logistic function in the fixed effects and to select only among the covariates. We did not include host social status among the covariates because it was unknown for Eurasian Reed Warblers and because there were no statistically significant differences in growth parameters of cuckoo nestlings reared by monogamous vs. polygynous Great Reed Warbler host males (Požgayová et al. 2015).
Model selection results for the effects of nestling sex (female, male), host species (Great Reed Warbler, Eurasian Reed Warbler), nestling hatching date, and their 2-way interactions on the growth of Common Cuckoo nestlings. In all models, the fixed effects took the form of a 3-parameter logistic function (fitted across all nestling masses and ages), which was extended by the respective covariates. Models with ΔAIC < 2 are highlighted in bold font. ΔAIC is the difference from the top model in Akaike's information criterion, log(L) is the log-likelihood of the model, and wi is the Akaike model weight.
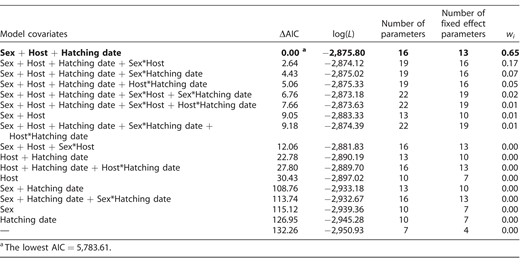
Model selection results for the effects of nestling sex (female, male), host species (Great Reed Warbler, Eurasian Reed Warbler), nestling hatching date, and their 2-way interactions on the growth of Common Cuckoo nestlings. In all models, the fixed effects took the form of a 3-parameter logistic function (fitted across all nestling masses and ages), which was extended by the respective covariates. Models with ΔAIC < 2 are highlighted in bold font. ΔAIC is the difference from the top model in Akaike's information criterion, log(L) is the log-likelihood of the model, and wi is the Akaike model weight.

In an additional analysis, we explored cuckoo fledging age (in days) in relation to sex, host species, and hatching date. For this purpose, we fit a set of generalized linear models wherein fledging age was treated as the dependent variable with a Poisson error distribution, and sex, host species, and hatching date were the predictor variables.
We selected all models based on Akaike's information criterion (AIC; Burnham and Anderson 2002). The set of models for the random effect structure comprised all possible combinations of the 3 growth parameters (correlated with each other or not; Table 1). The set of models with extended fixed effects included all combinations of covariates as well as their 2-way interactions (Table 2). The same initial model structure and selection procedure were applied when we explored fledging age (Appendix Table 4). Overall, models were ranked according to their AIC value and those with ΔAIC < 2 were selected as the most supported (Burnham and Anderson 2002). In the analysis of growth, only nestlings that survived at least until the age of 14 days were included (n = 121; males:females = 71:50). In the analysis of variation in fledging age, only successfully fledged nestlings could be considered (n = 66).
If not otherwise specified, we report means ± standard deviations (SD) for the data and 95% confidence limits (CLs) for test statistics throughout the text.
Results
Individual cuckoo nestlings varied in all 3 growth parameters and the random effect estimates of the parameters were tightly correlated (see the best-fitting model in Table 1). More specifically, there was a positive correlation between I and A of individual nestlings (r = 0.72 [0.61, 0.80]) and there were negative correlations between both K and A (r = −0.68 [−0.79, −0.51]) and K and I (r = −0.92 [−0.97, −0.82]). The strong correlation between K and I, however, implied that the best-fitting model was overparameterized (see Sofaer et al. 2013, Aldredge 2016). For further analyses, therefore, we used the random effect structure from the second-best model (Table 1).
Although the sexes did not differ in their hatching mass (3.07 ± 0.43 g and 3.11 ± 0.54 g for males and females, respectively; Figure 1), models with covariates revealed sex-specific differences in growth patterns (Table 2 and Figure 2). Apart from sex, the best-fitting model included host species and nestling hatching date (Table 2). No interactions between covariates received much support. The top model showed that males reached higher A and achieved I later than females (Table 3). In addition, cuckoo nestlings reared by Eurasian Reed Warblers achieved lower A and reached I earlier than nestlings reared by Great Reed Warblers (Table 3). The third growth parameter, K, was similar for both sexes, but it differed with host species: Nestlings reared by Eurasian Reed Warblers exhibited slightly higher K than those reared by Great Reed Warblers (Table 3). Nestling random effect for A explained more variation than nestling random effect for I (estimated SD for A = 6.61 [5.76, 7.59] and for I = 0.51 [0.43, 0.61]; residual: 2.61 [2.49, 2.74]). As a consequence of sex-specific growth, males showed higher mass before fledging (78.01 ± 10.39 g, measured at the age of 17–18 days) than females (71.06 ± 11.09 g; Welch two sample t-test: t79.2 = −3.27, P = 0.002).
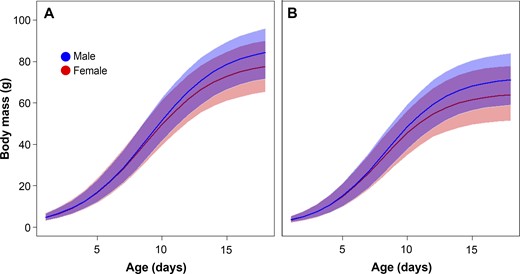
Fitted sex-specific growth curves of Common Cuckoo nestlings reared by (A) Great Reed Warblers and (B) Eurasian Reed Warblers. Growth curves are predicted from the best-fitting model with covariates (Table 3); shaded areas depict the variability among nestlings (95% credible intervals).
Estimates of the fixed effects, with 95% confidence limits (CL), from the best-fitting model (Table 2) explaining variation in Common Cuckoo nestling growth in relation to sex (female, male), host species (Great Reed Warbler, Eurasian Reed Warbler), and hatching date. Intercepts represent population means; sex- and host-specific estimates are in the form of deviation contrasts that compare the mean for a given level with the population mean. A = asymptotic mass (g), K = growth rate constant (day−1), I = inflection point of the growth curve (days). The predictors with 95% CL that do not include zero are highlighted in bold font.
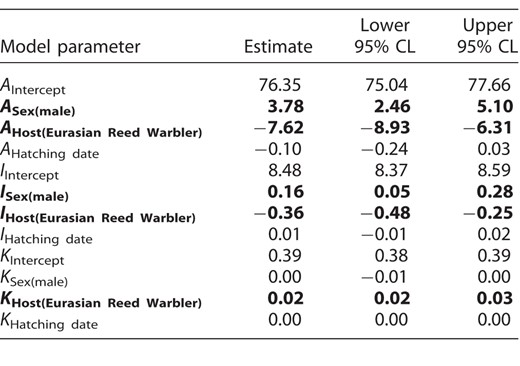
Estimates of the fixed effects, with 95% confidence limits (CL), from the best-fitting model (Table 2) explaining variation in Common Cuckoo nestling growth in relation to sex (female, male), host species (Great Reed Warbler, Eurasian Reed Warbler), and hatching date. Intercepts represent population means; sex- and host-specific estimates are in the form of deviation contrasts that compare the mean for a given level with the population mean. A = asymptotic mass (g), K = growth rate constant (day−1), I = inflection point of the growth curve (days). The predictors with 95% CL that do not include zero are highlighted in bold font.

Finally, neither sex nor other predictors reliably explained variation in fledging age (Appendix Tables 4 and 5).
Discussion
We found that male Common Cuckoo nestlings had no apparent head start in body mass on the day of hatching compared with female nestlings. Despite this, males were estimated to attain ∼8 g (10%) higher asymptotic mass (A) and similarly higher mass before fledging than females. Males, however, reached the point of maximum growth (I) only less than half a day later than females. Therefore, male and female growth constants (K) were almost identical, which resulted in no sex-specific differences in fledging age. Generally, these results agree well with growth patterns described for many sexually size-dimorphic species, wherein sexes do not differ in hatching mass, but one sex fledges at a higher mass than the other (Ricklefs 1968, Richner 1991, Teather and Weatherhead 1994). Also, it usually takes longer for the larger sex to reach its final size than the smaller sex, which appears to mature faster.
In the Brown-headed Cowbird, nestling sex was the most important predictor of growth rate, as males grew faster and fledged with higher mass than females, which might be explained by their better competitive ability when raised together with host young (Tonra et al. 2008). In contrast, Weatherhead (1989) found that the sexual size dimorphism seen in adult Brown-headed Cowbirds was not expressed in their nestlings, because there were no differences in growth rate and average nestling mass at the age of 7 days. In other species of parasitic cowbirds, namely the Shiny Cowbird (Molothrus bonariensis) and Screaming Cowbird (Molothrus rufoaxillaris), males grew at a faster rate and reached higher asymptotic mass than females (De Mársico et al. 2010, Tuero et al. 2013). Interestingly, before fledging, cuckoo and cowbird nestlings only approached the mass of adults (in cuckoos it was ∼75% and ∼79% of male and female adult mass, respectively; see also Payne 2005; for cowbirds see the references above). This suggests that brood parasitic nestlings still grow for some time after fledging before they reach adult size (as is known for many other species).
Because growth is energetically demanding, both the total mass gained during the period of development and the duration of this period may affect nestling energy requirements. Nestlings of the larger sex are thus expected to consume more food and be energetically more costly for parents to nurture than nestlings of the smaller sex, though this is not a general rule (see Magrath et al. 2007 and references therein). The higher asymptotic mass and mass before fledging detected in male cuckoo nestlings compared with female nestlings imply that males may have somewhat higher needs than females. To meet these needs, males may receive more (or higher quality) food from their foster parents than females. Nevertheless, in cuckoos raised by Great Reed Warblers, males did not seem to receive more food than females (M. Požgayová personal observation), which may be explained by the fact that they do not differ in vocal begging behavior from females (Abraham et al. 2015). To determine whether cuckoo males generally obtain more (or higher quality) food than females, a wider spectrum of host species, differing in size or other life history traits, should be studied. Another (and perhaps a better) possibility to find out if males indeed require more energy during growth might be to measure direct male and female metabolic activity (see, e.g., Magrath et al. 2007).
Alternatively, both sexes may receive the same amount of food, but the larger sex may utilize it more effectively and thus gain more mass (Magrath et al. 2007). High physiological activity can be stimulated by high levels of particular hormones, namely androgen steroids. There is some evidence of differences between the sexes in concentrations of egg yolk androgens and their positive effect on growth (reviewed by Gil 2008, von Engelhardt and Groothuis 2011). However, support for sex-specific levels of plasma-circulating androgens is mixed (see table 2 in Fargallo et al. 2007) and little is known about their association with growth (Goymann et al. 2005, Fargallo et al. 2007). In contrast to other species, brood parasites are highly underexplored in this respect and we encourage researchers to turn their attention to this topic.
On the other hand, the smaller sex may not be able to gain the same amount of mass from the same amount of food as the larger sex because it utilizes a large proportion of its energy in other ways. In contrast to the larger sex, which may preferentially invest in gaining mass, the smaller sex may instead allocate energy to maturation of particular body structures (e.g., tissues and plumage) that enable successful fledging (Moss 1979, Bancroft 1984, Mainwaring et al. 2011, but see Schaadt and Bird 1993) or increase begging success (e.g., larger gape; Ortega and Cruz 1992, Mainwaring et al. 2012, but see Clark 1995). Contrary to the species explored in the above cited studies, cuckoo nestlings grow up alone in host nests as they typically evict all nest mates shortly after hatching. Whether, in the absence of sibling competition, female cuckoo nestlings invest preferentially in alternative traits (i.e. through differential growth) and males preferentially allocate their energy to gaining mass remains open to future study. Regardless, an important factor that may play a role here is the tradeoff between resource allocation to growth vs. self-maintenance, which is mediated by oxidative stress (reviewed by Smith et al. 2016).
If the costs of raising a particular sex differ due to sexual size dimorphism, the more expensive (i.e. larger) sex may experience higher mortality than the less expensive one to raise. Indeed, there is a growing body of evidence that nestlings of the larger sex survive less well than nestlings of the smaller sex (reviewed by Kalmbach and Benito 2007, but see, e.g., Drummond et al. 1991, Weatherhead and Dufour 2005). As causes of nestling mortality other than predation are extremely scarce in our study population, we do not have enough data to test the hypothesis that heavier male cuckoo nestlings suffer more from starvation than female nestlings. Nonetheless, the markedly impaired growth of cuckoos raised by the small Eurasian Reed Warbler confirms the result of Kleven et al. (1999) that this host is of lower quality than the larger Great Reed Warbler.
Acknowledgments
We thank M. M. Abraham, R. Beňo, V. Brlík, M. Čapek, V. Jelínek, T. Karasová, J. Koleček, L. Kulísek, R. Poláková, B. Prudík, K. Sosnovcová, P. Steidlová, M. Šulc, and K. Žabková for their invaluable help in the field. We are grateful to the management of the Hodonín Fish Farm for permission to conduct the fieldwork on their grounds. We are also obliged to local nature conservation authorities for allowing us to work on protected animals.
Funding statement: This study was supported by the Czech Science Foundation (grant 17-12262S to M.H.). The funder had no influence on the content of the manuscript and required no permission before its submission or publication.
Ethics statement: This study was carried out with the permission of the regional nature conservation authorities (permit numbers JMK 115874/2013 and 38506/2016, and MUHOCJ 41433/2012/OŽP, 34437/2014/OŽP, and 14306/2016/OŽP). The fieldwork adhered to the animal care protocol (experimental project numbers 039/2011 AV ČR and 3030/ENV/17-169/630/17) and to the Czech Law on the Protection of Animals against Mistreatment (license numbers CZ 01272 and CZ 01284).
Author contributions: M.P. designed the study, conducted the fieldwork, analyzed the data, and wrote the paper; R.P. carried out the lab work and commented on the manuscript; M.H. ensured funding, supervised the research, and contributed to writing the paper; and P.P. collected and analyzed the data, and substantially edited the paper.
Literature Cited
Model selection results for the effect of nestling sex (female, male), host species (Great Reed Warbler, Eurasian Reed Warbler), hatching date, and their 2-way interactions on Common Cuckoo fledging age. Models with ΔAIC < 2 are highlighted in bold font. ΔAIC is the difference from the top model in Akaike's information criterion, log(L) is the log-likelihood of the model, and wi is the Akaike model weight.
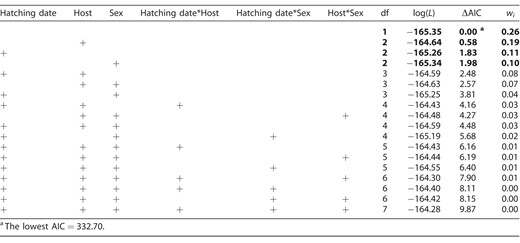
Model selection results for the effect of nestling sex (female, male), host species (Great Reed Warbler, Eurasian Reed Warbler), hatching date, and their 2-way interactions on Common Cuckoo fledging age. Models with ΔAIC < 2 are highlighted in bold font. ΔAIC is the difference from the top model in Akaike's information criterion, log(L) is the log-likelihood of the model, and wi is the Akaike model weight.

Averaged estimates of fixed effects (treatment contrasts), with 95% confidence limits (CL), from the most-supported models (ΔAIC < 2; Appendix Table 4) explaining variation in Common Cuckoo fledging age in relation to sex (female, male), host species (Great Reed Warbler, Eurasian Reed Warbler), and hatching date. The predictors with 95% CL that do not include zero are highlighted in bold font.
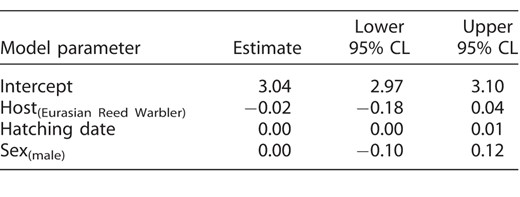
Averaged estimates of fixed effects (treatment contrasts), with 95% confidence limits (CL), from the most-supported models (ΔAIC < 2; Appendix Table 4) explaining variation in Common Cuckoo fledging age in relation to sex (female, male), host species (Great Reed Warbler, Eurasian Reed Warbler), and hatching date. The predictors with 95% CL that do not include zero are highlighted in bold font.