Abstract
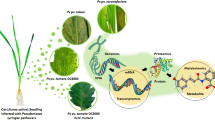
Medicinal plants have been used by marginal communities to treat various ailments. However, the potential of endophytes within these bio-prospective medicinal plants remains unknown. The present study elucidates the endophytic diversity of medicinal plants (Caralluma acutangula, Rhazya stricta, and Moringa peregrina) and the endophyte role in seed growth and oxidative stress. Various organs of medicinal plants yielded ten endophytes, which were identified as Phoma sp. (6 isolates), Alternaria sp. (2), Bipolaris sp. (1), and Cladosporium sp. (1) based on 18S rDNA sequencing and phylogenetic analysis. The culture filtrates (CFs; 25%, 50%, and 100% concentrations) from these endophytes were tested against the growth of normal and dwarf mutant rice lines. Endophytic CF exhibited dose-dependent growth stimulation and suppression effects. CF (100%) of Phoma sp. significantly increased rice seed germination and growth compared to controls and other endophytes. This growth-promoting effect was due to the presence of indole acetic acid in endophytic CF. The gas chromatography/mass spectrometry (GC/MS) analysis showed the highest indole acetic acid content ((54.31±0.21) μmol/L) in Bipolaris sp. In addition, the isolate of Bipolaris sp. exhibited significantly higher radical scavenging and anti-lipid peroxidation activity than the other isolates. Bipolaris sp. and Phoma sp. also exhibited significantly higher flavonoid and phenolic contents. The medicinal plants exhibited the presence of bio-prospective endophytic strains, which could be used for the improvement of crop growth and the mitigation of oxidative stresses.
摘要
目的
探讨药用植物内生菌的多样性及其在种子生长和氧化应激中的作用。
方法
从三种药用植物(Caralluma acutangula、Rhazya stricta 和 Moringa peregrina)中提取内生菌;基 于18S rDNA 测序和系统发育分析鉴定分离得到 的内生菌株;以正常和矮化突变体水稻品系为对 照,比较不同浓度的内生菌培养滤液(CF)对水 稻种子的发芽和生长的影响;通过气相色谱-质谱 分析CF 中的有效活性成分。
结论
从药用植物中共获得10 种内生菌,包括茎点霉 属6 株、链格孢属2 株、双极霉属1 株和枝孢霉 属1 株。CF 表现出剂量依赖性的生长刺激和抑制 作用。与对照和其他内生菌相比,100%的茎点霉 菌CF 显著促进了水稻种子的发芽和生长;双极 霉中的吲哚乙酸含量最高,并表现出比其更高的 自由基清除和抗脂质过氧化活性;双极霉菌和茎 点霉菌的类黄酮和酚类成分较高。综上所述,药 用植物中存在内生菌株,其可以用于改善作物生 长和减轻氧化应激。
Similar content being viewed by others
References
Ansari, M.W., Trivedi, D.K., Sahoo, R.K., et al., 2013. A critical review on fungi mediated plant responses with special emphasis to Piriformospora indica on improved production and protection of crops. Plant Physiol. Biochem., 70:403–410. http://dx.doi.org/10.1016/j.plaphy.2013.06.005
Arnold, A.E., Lutzoni, F., 2007. Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology, 88(3):541–549. http://dx.doi.org/10.1890/05-1459
Bajpai, V.K., Agrawal, P., Park, Y.H., 2014, Phytochemicals, antioxidant and anti-lipid peroxidation activities of ethanolic extract of a medicinal plant, Andrographis paniculata. J. Food Biochem., 38(6):584–591. http://dx.doi.org/10.1111/jfbc.12092
Chen, J., Hu, K.X., Hou, X.Q., et al., 2011. Endophytic fungi assemblages from 10 Dendrobium medicinal plants (Orchidaceae). World J. Microbiol. Biotechnol., 27(5):1009–1016. http://dx.doi.org/10.1007/s11274-010-0544-y
Chutima, R., Lumyong, S., 2012. Production of indole-3-acetic acid by Thai native orchid-associated fungi. Symbiosis, 56(1):35–44. http://dx.doi.org/10.1007/s13199-012-0158-2
de Hoog, G.S., Horre, R., 2002. Molecular taxonomy of the Alternaria and Ulocladium species from humans and their identification in the routine laboratory. Mycoses, 45(7-8): 259–276. http://dx.doi.org/10.1046/j.1439-0507.2002.00747.x
Ding, X., Liu, K., Deng, B., et al., 2013. Isolation and characterization of endophytic fungi from Camptotheca acuminata. World J. Microbiol. Biotechnol., 29(10):1831–1838. http://dx.doi.org/10.1007/s11274-013-1345-x
Gao, J.M., Xiao, J., Zhang, Q., et al., 2014. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem., 62(16):3584–3590. http://dx.doi.org/10.1021/jf500054f
Garcia, A., Rhoden, S.A., Rubin-Filho, C.J., et al., 2012. Diversity of foliar endophytic fungi from the medicinal plant Sapindus saponaria L. and their localization by scanning electron microscopy. Biol. Res., 45(2):139–148. http://dx.doi.org/10.4067/S0716-97602012000200006
Ghimire, S.R., Charlton, N.D., Bell, J.D., et al., 2011. Biodiversity of fungal endophyte communities inhabiting switchgrass (Panicum virgatum L.) growing in the native tall grass prairie of northern Oklahoma. Fungal Div., 47(1):19–27. http://dx.doi.org/10.1007/s13225-010-0085-6
Ghosh, S., Derle, A., Ahire, M., et al., 2013. Phytochemical analysis and free radical scavenging activity of medicinal plants Gnidia glauca and Dioscorea bulbifera. PLoS ONE, 8(12):e82529. http://dx.doi.org/10.1371/journal.pone.0082529
Gubiani, J.R., Zeraik, M.L., Oliveira, C.M., et al., 2014. Biologically active eremophilane-type sesquiterpenes from Camarops sp., an endophytic fungus isolated from Alibertia macrophylla. J. Nat. Prod., 77(3):668–672. http://dx.doi.org/10.1021/np400825s
Gulati, V., Harding, I.H., Palombo, E.A., 2012. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: potential application in the management of hyperglycemia. BMC Comp. Alter. Med., 12:77. http://dx.doi.org/10.1186/1472-6882-12-77
Hilbert, M., Voll, L.M., Ding, Y., et al., 2012. Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol., 196(2): 520–534. http://dx.doi.org/10.1111/j.1469-8137.2012.04275.x
Huang, W.Y., Cai, Y.Z., Xing, J., et al., 2007. A potential antioxidant resource: endophytic fungi from medicinal plants. Econ. Bot., 61(1):14–30. http://dx.doi.org/10.1663/0013-0001(2007)61[14:APARE F]2.0.CO;2
Keerthi, M., Jumpponen, A., 2014. Unraveling the Dark Septate Endophyte Functions: Insights from the Arabidopsis Model. Advances in Endophytic Research. Springer India, p.115–141.
Kende, H., 2001. Hormone response mutants: a plethora of surprises. Plant Physiol., 125(1):81–84. http://dx.doi.org/10.1104/pp.125.1.81
Khan, A.L., Hamayun, M., Kim, Y.H., et al., 2011. Ameliorative symbiosis of endophyte (Penicillium funiculosum LHL06) under salt stress elevated plant growth of Glycine max L. Plant Physiol. Biochem., 49(8):852–862. http://dx.doi.org/10.1016/j.plaphy.2011.03.005
Khan, A.L., Hussain, J., Al-Harrasi, A., et al., 2013. Endophytic fungi: a source of gibberellins and crop resistance to abiotic stress. Crit. Rev. Biotech., 35(1):1–13. http://dx.doi.org/10.3109/07388551.2013.800018
Kipkore, W., Wanjohi, B., Rono, H., et al., 2014. A study of the medicinal plants used by the Marakwet Community in Kenya. J. Ethnobiol. Ethnomed., 10(1):24. http://dx.doi.org/10.1186/1746-4269-10-24
Kumar, D.S.S., Hyde, K.D., 2004. Biodiversity and tissuerecurrence of endophytic fungi in Tripterygium wilfordii. Fungal Divers., 17:69–90.
Kusari, S., Singh, S., Jayabaskaran, C., 2014. Biotechnological potential of plant-associated endophytic fungi: hope versus hype. Trends Biotechnol., 32(6):297–303. http://dx.doi.org/10.1016/j.tibtech.2014.03.009
Liu, X., Dong, M., Chen, X., et al., 2007. Antioxidant activity and phenolics of an endophytic Xylaria sp. from Ginkgo biloba. Food Chem., 105(2):548–554. http://dx.doi.org/10.1016/j.foodchem.2007.04.008
Mandyam, K.G., Roe, J., Jumpponen, A., 2013. Arabidopsis thaliana model system reveals a continuum of responses to root endophyte colonization. Fungal Biol., 117(4): 250–260. http://dx.doi.org/10.1016/j.funbio.2013.02.001
Murphy, B.R., Doohan, F.M., Hodkinson, T.R., 2014. Yield increase induced by the fungal root endophyte Piriformospora indica in barley grown at low temperature is nutrient limited. Symbiosis, 62(1):29–39. http://dx.doi.org/10.1007/s13199-014-0268-0
Nalini, M.S., Sunayana, N., Prakash, H.S., 2014. Endophytic fungal diversity in medicinal plants of Western Ghats, India. Int. J. Biodiv., 2014:1–9. http://dx.doi.org/10.1155/2014/494213
Nath, A., Chattopadhyay, A., Joshi, S.R., 2015. Biological activity of endophytic fungi of Rauwolfia serpentine Benth: an ethnomedicinal plant used in folk medicines in northeast India. PNAS, 85(1):233–240. http://dx.doi.org/10.1007/s40011-013-0184-8
Nishijima, T., Koshioka, M., Yamazaki, H., 1994. Use of several gibberellin biosynthesis inhibitors in sensitized rice seedling bioassays. Biosci. Biotechnol. Biochem., 58(3):572–573. http://dx.doi.org/10.1271/bbb.58.572
Orlandelli, R.C., Alberto, R.N., Rubin Filho, C.J., et al., 2012. Diversity of endophytic fungal community associated with Piper hispidum (Piperaceae) leaves. Genet. Mol. Res., 11(2):1575–1585. http://dx.doi.org/10.4238/2012.May.22.7
Rai, M.R., Rathod, D., Agarkar, G., et al., 2014. Fungal growth promotor endophytes: a pragmatic approach towards sustainable food and agriculture. Symbiosis, 62(2):63–79. http://dx.doi.org/10.1007/s13199-014-0273-3
Redman, R.S., Kim, Y.O., Woodward, C.J.D.A., et al., 2011. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: a strategy for mitigating impacts of climate change. PLoS ONE, 6(7):e14823. http://dx.doi.org/10.1371/journal.pone.0014823
Reinhardt, D., Mandel, T., Kuhlemeier, C., 2000. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell, 12(4):507–518. http://dx.doi.org/10.2307/3871065
Saeed, N., Khan, M.R., Shabbir, M., 2012. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Comp. Alter. Med., 12:221. http://dx.doi.org/10.1186/1472-6882-12-221
Sakayaroj, Y., Preedanon, S., Supaphon, O., et al., 2010. Phylogenetic diversity of endophyte assemblages associated with the tropical seagrass Enhalus acoroides in Thailand. Fungal Div., 42(1):27–45. http://dx.doi.org/10.1007/s13225-009-0013-9
Schulz, B., Boyle, C., 2005. The endophytic continuum. Mycol. Res., 109(6):661–686. http://dx.doi.org/10.1017/S095375620500273X
Slinkard, K., Singleton, L., 1977. Total phenol analyses: automation and comparison with manual methods. Am. J. Enol. Vitic., 28:49–55.
Strobel, G., Daisy, B., Castillo, U., et al., 2004. Natural products from endophytic microorganisms. J. Nat. Prod., 67(2):257–268. http://dx.doi.org/10.1021/np030397v
Tamura, K., Peterson, D., Peterson, N., et al., 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol., 28(10):2731–2739. http://dx.doi.org/10.1093/molbev/msr121
Tang, A.M.C., Jeewon, R., Hyde, K.D., 2009. A re-evaluation of the evolutionary relationships within the Xylariaceae based on ribosomal and protein-coding gene sequences. Fungal Div., 34:127–155.
Thakur, A., Kaur, S., Kaur, A., et al., 2013. Enhanced resistance to Spodoptera litura in endophyte infected cauliflower plants. Environ. Entomol., 42(2):240–246. http://dx.doi.org/10.1603/EN12001
Torres, M.S., White, J.F.Jr., Zhang, X., et al., 2012. Endophytemediated adjustments in host morphology and physiology and effects on host fitness traits in grasses. Fungal Ecol., 5(3):322–330. http://dx.doi.org/10.1016/j.funeco.2011.05.006
Ullah, I., Khan, A.R., Park, G.S., et al., 2013. Analysis of phytohormones and phosphate solubilization in Photorhabdus spp. Food Sci. Biotechnol., 22(S1):25–31. http://dx.doi.org/10.1007/s10068-013-0044-6
Vadassery, V., Ritter, C., Venus, Y., et al., 2008. The role of auxins and cytokinins in the mutualistic interaction between Arabidopsis and Piriformospora indica. MPMI, 21(10):1371–1383. http://dx.doi.org/10.1094/MPMI-21-10-1371
Waller, F., Achatz, B., Baltruschat, H., et al., 2005. The endophytic fungus Piriformis indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. PNAS, 102(38):13386–13391. http://dx.doi.org/10.1073/pnas.0504423102
Wang, L.W., Xu, B.G., Wang, J.Y., et al., 2012. Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens. Appl. Microbiol. Biotechnol., 93(3):1231–1239. http://dx.doi.org/10.1007/s00253-011-3472-3
Waqas, M., Khan, A.L., Lee, I.J., 2014. Bioactive chemical constituents produced by endophytes and effects on rice plant growth. J. Plant Interact., 9(1):478–487. http://dx.doi.org/10.1080/17429145.2013.860562
White, J.F. Jr., Torres, M.S., 2010. Is plant endophyte mediated defensive mutualism the result of oxidative stress protection? Physiol. Plant., 138(4):440–446. http://dx.doi.org/10.1111/j.1399-3054.2009.01332.x
Xiao, J., Zhang, Q., Gao, Y.Q., et al., 2014. Secondary metabolites from the endophytic Botryosphaeria dothidea of Melia azedarach and their antifungal, antibacterial, antioxidant, and cytotoxic activities. J. Agric. Food Chem., 62(16):3584–3590. http://dx.doi.org/10.1021/jf500054f
Zhang, H., Xiong, H., Zhao, H., et al., 2013. An antimicrobial compound from the endophytic fungus Phoma sp. isolated from the medicinal plant Taraxacum mongolicum. J. Taiwan Inst. Chem. Eng., 44(2):177–181. http://dx.doi.org/10.1016/j.jtice.2012.11.013
Zhang, Y., Crous, P.W., Schoch, C.L., et al., 2011. Pleosporales. Fungal Div., 53(1):1–221. http://dx.doi.org/10.1007/s13225-011-0117-x
Zhao, J.T., Ma, D.H., Luo, M., et al., 2014. In vitro antioxidant activities and antioxidant enzyme activities in HepG2 cells and main active compounds of endophytic fungus from pigeon pea [Cajanus cajan (L.) Millsp.]. Food Res. Int., 56:243–251. http://dx.doi.org/10.1016/j.foodres.2013.12.028
Author information
Authors and Affiliations
Corresponding authors
Additional information
Project supported by the Oman Research Council (FURAP Program), and the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agriculture, Food and Rural Affairs Research Center Support Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (716001-7)
Electronic supplementary materials: The online version of this article (http://dx.doi.org/10.1631/jzus.B1500271) contains supplementary materials, which are available to authorized users
ORCID: Ahmed AL-HARRASI, http://orcid.org/0000-0002-0815-5942
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Khan, A.L., Gilani, S.A., Waqas, M. et al. Endophytes from medicinal plants and their potential for producing indole acetic acid, improving seed germination and mitigating oxidative stress. J. Zhejiang Univ. Sci. B 18, 125–137 (2017). https://doi.org/10.1631/jzus.B1500271
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1500271