Abstract
Toxoplasmosis, a disease caused by Toxoplasma gondii, is an important health problem, especially in immunocompromised hosts. T. gondii uses the gut wall as an infection gateway, with tropism for muscular and nervous tissues causing intestinal alterations, including some in the enteric nervous system. This study aims at investigating the colon of rats infected by T. gondii in order to understand how the amount of oocysts influences in myenteric neuronal changes. Sixty Wistar rats (Rattus norvegicus) were divided into six groups. One group remained as a control and the others received inocula of 10, 50, 100, 500 or 5,000 oocysts of T. gondii. The animals were euthanized after 30 days of infection. The total neuronal population and the nitrergic subpopulation in the colon myenteric plexus of each animal was counted. The data were statistically analyzed showing less weight gain in rats with 10, 500 and 5,000 oocysts. A decrease in the number of total neurons with 50, 100 or 5,000 oocysts and an increase in the nitrergic population with 10, 100, 500 or 5,000 oocysts were verified. These results show that neuronal alterations are more significant when the infection is induced by larger inocula and reinforces the suspicion that neuronal loss is directed at cholinergic neurons.
Keywords:
Enteric nervous system; myenteric plexus; ascending colon; toxoplasmosis
Resumo
A toxoplasmose, doença causada pelo Toxoplasma gondii, é um importante problema de saúde, principalmente em imunocomprometidos. T. gondii utiliza a parede do intestino como porta de entrada no hospedeiro e tem tropismo pelos tecidos muscular e nervoso provocando alterações intestinais, inclusive no sistema nervoso entérico. Este estudo buscou analisar o cólon de ratos infectados por T. gondii para entender como a quantidade de oocistos influencia nas alterações neuronais mientéricas. Foram utilizados 60 ratos Wistar (Rattus norvegicus) em seis grupos. Um dos grupos permaneceu como controle e os demais receberam inóculos de 10, 50, 100, 500 ou 5.000 oocistos de T. gondii. Os animais foram submetidos a eutanásia após 30 dias de infecção. No plexo mientérico do cólon dos animais foram quantificadas a população neuronal total e a subpopulação nitrérgica. Os dados foram analisados estatisticamente demonstrando inferior ganho de peso nos ratos com 10, 500 e 5.000 oocistos. Verificamos diminuição no número de neurônios totais com inóculos de 50, 100 ou 5.000 oocistos e aumento da população nitrérgica com 10, 100, 500 ou 5000 oocistos. Estes resultados mostram que alterações neuronais são mais significativas quando a infecção é induzida por inóculos maiores e reforça a suspeita de perda neuronal direcionada a neurônios colinérgicos.
Palavras-chave:
Sistema nervoso entérico; plexo mientérico; cólon ascendente; toxoplasmose
Introduction
Toxoplasma gondii is an obligate intracellular coccidian protozoan parasite, which is widely distributed (WEISS & KIM, 2007Weiss L, Kim K. Toxoplasma gondii: the model apicomplexan: perspectives and methods. Rio de Janeiro: Elsevier; 2007.). In Brazil, seroepidemiological studies show the presence of antibodies against T. gondii in about 65% of the population (FEREZIN et al., 2013Ferezin RI, Bertolini DA, Demarchi IG. Prevalência de sorologia positiva para HIV, hepatite B, toxoplasmose e rubéola em gestantes do noroeste paranaense. Rev Bras Ginecol Obstet 2013; 35(2): 66-70. PMid:23412005. http://dx.doi.org/10.1590/S0100-72032013000200005.
http://dx.doi.org/10.1590/S0100-72032013...
; REICHE et al., 2000Reiche EMV, Morimoto HK, Farias GN, Hisatsugu KR, Geller L, Gomes ACLF, et al. Prevalência de tripanossomíase americana, sífilis, toxoplasmose, rubéola, hepatite B, hepatite C e da infecção pelo vírus da imunodeficiência humana, avaliada por intermédio de testes sorológicos, em gestantes atendidas no período de 1996 a 1998 no Hospital Universitário Regional Norte do Paraná (Universidade Estadual de Londrina, Paraná, Brasil). Rev Soc Bras Med Trop 2000; 33(6): 519-527. PMid:11175581. http://dx.doi.org/10.1590/S0037-86822000000600002.
http://dx.doi.org/10.1590/S0037-86822000...
; SARTORI et al., 2011Sartori AL, Minamisava R, Avelino MM, Martins CA. Triagem pré-natal para toxoplasmose e fatores associados à soropositividade de gestantes em Goiânia, Goiás. Rev Bras Ginecol Obstet 2011; 33(2): 93-98. PMid:21779652. http://dx.doi.org/10.1590/S0100-72032011000200007.
http://dx.doi.org/10.1590/S0100-72032011...
). The definitive hosts in the life cycle of T. gondii are cats and other felids, and several animal species such as birds and mammals act as intermediate hosts (DUBEY, 2007Dubey JP. The history and life cycle of . In: Toxoplasma gondiiWeiss L, Kim K. Toxoplasma gondii: the model apicomplexan: perspectives and methods. Rio de Janeiro: Elsevier; 2007. p. 1-17.). In humans, its main transmission probably occurs through the ingestion of water or food contaminated with oocysts (MONTOYA & LIESENFELD, 2004Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004; 363(9425): 1965-1976. PMid:15194258. http://dx.doi.org/10.1016/S0140-6736(04)16412-X.
http://dx.doi.org/10.1016/S0140-6736(04)...
; WEISS & KIM, 2007Weiss L, Kim K. Toxoplasma gondii: the model apicomplexan: perspectives and methods. Rio de Janeiro: Elsevier; 2007.). After this, the oocysts release the sporozoites, upon crossing the intestinal wall, change into their infective form, tachyzoites, which reach the bloodstream (DUBEY, 2007Dubey JP. The history and life cycle of . In: Toxoplasma gondiiWeiss L, Kim K. Toxoplasma gondii: the model apicomplexan: perspectives and methods. Rio de Janeiro: Elsevier; 2007. p. 1-17.).
The pathological process of toxoplasmosis depends on the parasite genotype. One of the major and classical genetic classifications of T. gondii strains in Europe and USA comes from the SAG2 gene analysis (HOWE & SIBLEY, 1995Howe DK, Sibley LD. comprises three clonal lineages: correlation of parasite genotype with human disease. Toxoplasma gondiiJ Infect Dis 1995; 172(6): 1561-1566. PMid:7594717. http://dx.doi.org/10.1093/infdis/172.6.1561.
http://dx.doi.org/10.1093/infdis/172.6.1...
). The type I strain is considered of high virulence, type II is of intermediate virulence and type III is of low virulence (SILVA et al., 2005Silva AV, Pezerico SB, Lima VY, d’Arc Moretti L, Pinheiro JP, Tanaka EM, et al. Genotyping of strains isolated from dogs with neurological signs. Toxoplasma gondiiVet Parasitol 2005; 127(1): 23-27. PMid:15619371. http://dx.doi.org/10.1016/j.vetpar.2004.08.020.
http://dx.doi.org/10.1016/j.vetpar.2004....
; FREYRE et al., 2001Freyre A, Falcón J, Correa O, Mendez J, González M, Venzal JM. Residual infection of 15 toxoplasma strains in the brain of rats fed cysts. Parasitol Res 2001; 87(11): 915-918. PMid:11728015.). The strain used in this study, identified as ME49 (genotype II), was isolated, in the northern hemisphere, from the muscles of a ram (LUNDE & JACOBS, 1983Lunde MN, Jacobs L. Antigenic differences between endozoites and cystozoites of Toxoplasma gondii.J Parasitol 1983; 69(5): 806-808. PMid:6200590. http://dx.doi.org/10.2307/3281034.
http://dx.doi.org/10.2307/3281034...
), and the fact that it is not of high virulence favors its use in studies of the chronic phase of the infection (MAUBON et al., 2008Maubon D, Ajzenberg D, Brenier-Pinchart MP, Dardé ML, Pelloux H. What are the respective host and parasite contributions to toxoplasmosis? Trends Parasitol 2008; 24(7): 299-303. PMid:18514029. http://dx.doi.org/10.1016/j.pt.2008.03.012.
http://dx.doi.org/10.1016/j.pt.2008.03.0...
).
In the chronic phase of the disease, the protozoan tends to form tissue cyst tropism in the nervous and muscle tissues. The intestinal wall is the first barrier it transposes to infect the host and then to lodge in these sites (DUBEY et al., 1998Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev 1998; 11(2): 267-299. PMid:9564564.). Previous studies of our research group focused on the changes caused by Toxoplasma gondii infection in the enteric nervous system (ENS), which is responsible for controlling important functions of the gastrointestinal tract (GIT) (BONAPAZ et al., 2010Bonapaz RS, Hermes-Uliana C, Santos FN, Silva AV, Araújo EJA, Sant’Ana DMG. Effects of infection with Toxoplasma gondii oocysts on the intestinal wall and myenteric plexus of chicken (). Gallus gallusPesqui Vet Bras 2010; 30(9): 787-792. http://dx.doi.org/10.1590/S0100-736X2010000900013.
http://dx.doi.org/10.1590/S0100-736X2010...
; ODORIZZI et al., 2010Odorizzi L, Moreira NM, Gonçalves GF, Silva AV, Sant’Ana DMG, Araújo EJA. Quantitative and morphometric changes of subpopulations of myenteric neurons in swines with toxoplasmosis. Auton Neurosci 2010; 155(1-2): 68-72. PMid:20167543. http://dx.doi.org/10.1016/j.autneu.2010.01.012.
http://dx.doi.org/10.1016/j.autneu.2010....
; PEREIRA et al., 2010Pereira LS, Silva AV, Araújo EJA, Sant’Ana DMG. Hypertrophy of NADH-diaphorase positive myenteric neurons in rat jejunum after acute infection caused by Toxoplasma gondii.J Venom Anim Toxins Incl Trop Dis 2010; 16(2): 298-310.; HERMES-ULIANA et al., 2011Hermes-Uliana C, Pereira-Severi LS, Luerdes RB, Franco CLM, Silva AV, Araújo EJA, et al. Chronic infection with causes myenteric neuroplasticity of the jejunum in rats. Toxoplasma gondiiAuton Neurosci 2011; 160(1-2): 3-8. PMid:20932812. http://dx.doi.org/10.1016/j.autneu.2010.09.003.
http://dx.doi.org/10.1016/j.autneu.2010....
; ARAÚJO et al., 2015Araújo EJA, Zaniolo LM, Vicentino SL, Góis MB, Zanoni JN, Silva AV, et al. causes death and plastic alteration in the jejunal myenteric plexus. Toxoplasma gondiiWorld J Gastroenterol 2015; 21(16): 4829-4839. PMid:25944996. http://dx.doi.org/10.3748/wjg.v21.i16.4829.
http://dx.doi.org/10.3748/wjg.v21.i16.48...
; GÓIS et al., 2016aGóis MB, Hermes-Uliana C, Zago MCB, Zanoni JN, Silva AV, Miranda MH No, et al. Chronic infection with induces death of submucosal enteric neurons and damage in the colonic mucosa of rats. Toxoplasma gondiiExp Parasitol 2016a; 164: 56-63. PMid:26902605. http://dx.doi.org/10.1016/j.exppara.2016.02.009.
http://dx.doi.org/10.1016/j.exppara.2016...
; TREVIZAN et al., 2016Trevizan AR, Vicentino-Vieira SL, Silva Watanabe P, Góis MB, de Melo GAN, Garcia JL, et al. Kinetics of acute infection with and histopathological changes in the duodenum of rats. Toxoplasma gondiiExp Parasitol 2016; 165: 22-29. PMid:26993084. http://dx.doi.org/10.1016/j.exppara.2016.03.015.
http://dx.doi.org/10.1016/j.exppara.2016...
).
The ENS consists of enteroglial cells and neurons distributed in two ganglionic plexuses: the myenteric plexus, which is located between the longitudinal and circular muscle layers, and mainly controls the contraction and the intestinal peristalsis; and the submucosal plexus, situated along the submucosa, responsible mainly for the regulation of mucus secretion and local blood flow (FURNESS, 2006Furness JB. The enteric nervous system. Malden: Blackwell; 2006.; GÓIS et al., 2016bGóis MB, Hermes-Uliana C, Paltanin A, Pontes W, Araújo EJA, Miranda MH No, et al. Morphoquantitative study of submucosal plexus by different neuronal evidentiation histochemical techniques. Rattus norvegicusInt J Morphol. 2016b; 34(4): 1487-1493.). Among the different neuronal populations within ENS, the nitrergic population comprises neurons with nitric oxide synthase type I activity (NOS-I), a NADPH-dependent enzyme involved in the synthesis of nitric oxide. Among its numerous functions, nitric oxide acts as a nerve cell transmitter, promoting the relaxation and inhibiting the contraction of the intestinal smooth muscle (DUSSE et al., 2003Dusse LMS, Vieira LM, Carvalho MG. Revisão sobre óxido nítrico. J Bras Patol Med Lab 2003; 39(4): 343-350. http://dx.doi.org/10.1590/S1676-24442003000400012.
http://dx.doi.org/10.1590/S1676-24442003...
; IYENGAR et al., 1987Iyengar R, Stuehr DJ, Marletta MA. Macrophage synthesis of nitrite, nitrate and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci USA 1987; 84(18): 6369-6373. PMid:2819872. http://dx.doi.org/10.1073/pnas.84.18.6369.
http://dx.doi.org/10.1073/pnas.84.18.636...
).
Recent reports indicate that rats infected with T. gondii present histological and immunological changes in the GI similar to those seen in inflammatory bowel diseases (LIESENFELD, 2002Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J Infect Dis 2002;185(Suppl 1): 96-101. PMid:11865446. http://dx.doi.org/10.1086/338006.
http://dx.doi.org/10.1086/338006...
; SCHREINER & LIESENFELD, 2009Schreiner M, Liesenfeld O. Small intestinal inflammation following oral infection with Toxoplasma gondii does not occur exclusively in C57BL/6 mice: review of 70 reports from the literature. Mem Inst Oswaldo Cruz 2009; 104(2): 221-233. PMid:19430647. http://dx.doi.org/10.1590/S0074-02762009000200015.
http://dx.doi.org/10.1590/S0074-02762009...
). Thus, the study of changes caused by T. gondii infection in the ENS may help shed light on the changes that occur in those pathological conditions. Although the colon is the main organ involved in inflammatory bowel diseases (MEYERS & JANOWITZ, 1989Meyers S, Janowitz HD. The “natural history” of ulcerative colitis: an analysis of the placebo response. J Clin Gastroenterol 1989; 11(1): 33-37. PMid:2646359. http://dx.doi.org/10.1097/00004836-198902000-00008.
http://dx.doi.org/10.1097/00004836-19890...
; BAUMGART & SANDBORN, 2012Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012; 380(9853): 1590-1605. PMid:22914295. http://dx.doi.org/10.1016/S0140-6736(12)60026-9.
http://dx.doi.org/10.1016/S0140-6736(12)...
), and is associated with the cause of intestinal tract disorders in various diseases, only a few studies have examined the relationship between T. gondii infection and local innervation. Sugauara et al. (2008)Sugauara EYY, Sant’Ana DMG, Almeida EC, Reis AB, Silva AV, Araújo EJA. Alterations of the myenteric plexus of the ileum and the descending colon caused by (genotype III). Toxoplasma gondiiArq Neuropsiquiatr 2008; 66(3A): 516-523. PMid:18813711. http://dx.doi.org/10.1590/S0004-282X2008000400015.
http://dx.doi.org/10.1590/S0004-282X2008...
found that rats infected with a Brazilian strain of T. gondii, BTU-II (genotype III), for 30 days exhibited myenteric neuron hypertrophy of the colon. Another study, which involved genotype I tachyzoites (BTU-IV strain), found an increase of cytoplasm in this neuronal population (SOARES et al., 2009Soares J, Moreira NM, Silva AV, Sant’Ana DMG, Araújo EJA. Infecção crônica por Toxoplasma gondii induzindo hipertrofia de neurônios do plexo mientérico do cólon descendente de Rattus norvegicus.Rev Bras Parasitol Vet 2009; 18(2): 57-60. PMid:19602320. http://dx.doi.org/10.4322/rbpv.01802013.
http://dx.doi.org/10.4322/rbpv.01802013...
). However, neither of the two aforementioned studies reported quantitative changes, and there are no reports of studies with the ME49 strain or any other of genotype II.
Therefore, the purpose of this study was to evaluate the changes caused by different inoculum loads of T. gondii on total myenteric and nitrergic neurons in rat colon, and to help consolidate a standard experimental model of infection with T. gondii that best represents its effects on the GIT.
Materials and Methods
The experimental protocol was approved by the Ethics Committee for Animal Experimentation of the State University of Maringá (Protocol No. 081/2012).
Experimental design
Sixty 60-day-old Wistar rats (Rattus norvegicus) were randomly distributed into six groups of 10 rats each. The control group (CG) received sterile saline orally by gavage, while the animals of the other groups were ranked according to the number of inoculated T. gondii oocysts (10, 50, 100, 500, or 5000). Inoculation was also performed orally, using the ME49 strain (genotype II) oocysts, previously sporulated and resuspended in 1 mL of sterile saline, which was obtained from the Department of Preventive Veterinary Medicine, State University of Londrina, Paraná, Brazil.
The animals were housed in individual cages for 30 days, with 12-hour light/dark cycles, under controlled temperature and humidity, and were fed rodent chow and water ad libitum. The animals were weighed on days 1 and 30 to calculate their body mass, and were observed during the experimental period for possible clinical signs of toxoplasmosis infection. The experimental design was entirely randomized.
Confirmation of infection with T. gondii
Thirty days after inoculation, blood samples were drawn from all the animals and subjected to a direct agglutination test to detect IgG antibodies against T. gondii, indicating immunity to the protozoan. Samples with a titer ≥ 1:25 were considered positive (DESMONTS & REMINGTON, 1980Desmonts G, Remington JS. Direct agglutination test for diagnosis of infection: method of increasing sensitivity and specificity. ToxoplasmaJ Clin Microbiol 1980; 11(6): 562-568. PMid:7000807.).
Euthanasia and sample collection
Thirty days after inoculation, the animals were euthanized by deep anesthesia with halothane vapor (VIVAS et al., 2007Vivas LAM, Jamel N, Refinetti RA, Silva LF, Rodrigues LV, Silva PC, et al. Anesthetic experimental device for small animal. Acta Cir Bras 2007; 22(3): 229-233. PMid:17546297. http://dx.doi.org/10.1590/S0102-86502007000300012.
http://dx.doi.org/10.1590/S0102-86502007...
). Each animal was laparotomized, followed by the removal and measurement of the ascending colon to calculate the area, and the removal of two segments of approximately 3 cm for subsequent histological processing.
Sample processing
The myenteric plexus was marked using two techniques: the pan-neuronal Giemsa staining technique (BARBOSA, 1978Barbosa AJA. Técnica histológica para gânglios nervosos intramurais em preparados espessos. Rev Bras Pesqui Med Biol 1978; 11(2-3): 95-97. PMid:80017.) and the NADPH-diaphorase (NADPH-d) histoenzymological technique, which marks the neuronal subpopulation with NOS-I activity (PHILLIPS et al., 2003Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci 2003; 106(2): 69-83. PMid:12878075. http://dx.doi.org/10.1016/S1566-0702(03)00072-9.
http://dx.doi.org/10.1016/S1566-0702(03)...
; SCHERER-SINGLER et al., 1983Scherer-Singler U, Vincent SR, Kimura H, McGeer EG. Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods 1983; 9(3): 229-234. PMid:6363828. http://dx.doi.org/10.1016/0165-0270(83)90085-7.
http://dx.doi.org/10.1016/0165-0270(83)9...
).
Giemsa technique
The segments were washed with a 0.9% NaCl solution and filled with formaldehyde-acetic fixative solution for at least 48 hours. The mucosa and submucosa were then removed using a stereomicroscope with trans-illumination to reveal the myenteric plexus, which was then stained with methylene blue in Sorensen’s phosphate buffer solution (pH = 7.0) for 24 hours (BARBOSA, 1978Barbosa AJA. Técnica histológica para gânglios nervosos intramurais em preparados espessos. Rev Bras Pesqui Med Biol 1978; 11(2-3): 95-97. PMid:80017.). The whole mounts were then dehydrated in a series of alcohol solutions of increasing concentration and cleared in xylol.
NADPH-diaphorase technique
The segments were washed and filled with 0.1M PBS (pH 7.3), fixed with 4.0% paraformaldehyde in 0.1M phosphate buffer for 30 minutes, and immersed in PBS with 0.3% Triton X-100 for 10 minutes. They were then washed in ten times in10-min baths in PBS for subsequent incubation for two hours in a medium containing the following ingredients in 200 mL: 100 mg of β-NADPH (Sigma, St. Louis, MO, USA), 0.6 mL of Triton X-100, and 50 mg of NBT in Tris-HCl solution (Life Technologies, Grand Island, NY) (SCHERER-SINGLER et al., 1983Scherer-Singler U, Vincent SR, Kimura H, McGeer EG. Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods 1983; 9(3): 229-234. PMid:6363828. http://dx.doi.org/10.1016/0165-0270(83)90085-7.
http://dx.doi.org/10.1016/0165-0270(83)9...
). The membrane preparations were then dehydrated in series of alcohol solutions of increasing concentration and cleared in xylol.
Quantitative analysis of myenteric neurons
All the myenteric neurons visible under 400X magnification in 120 fields of an optical microscope (Motic) were counted. The number of neurons of intestinal area was calculated based on the measurement of the microscopic field (0.249 mm2). The same procedure was performed for both preparation techniques in all the animals.
Statistical analysis
The sample residues were submitted to the Shapiro-Wilk test to verify distribution type (ZAR, 1999Zar JH. Biostatistical analysis. 4th. ed. New Jersey: Prentice Hall; 1999.). Data with normal distribution were expressed by mean ± SDs. Results of the neuron counts analysis were compared between groups using one-way analysis of variance (ANOVA) and Dunnett's multiple comparison post hoc test. The statistical analysis of the data was performed using Graph Pad Prism 5.01 software. For all statistic tests, p-values less than 0.05 were considered significant.
Results
The animals of the CG showed negative IgG serology against T. gondii, while the animals inoculated with the parasite presented positive serology, albeit without obvious clinical signs of toxoplasmosis, such as changes in the intestinal tract. The animals of the infected groups, G10, G500 and G5000, presented lower body mass gain than the CG (p<0.05), although the colon area in all the groups showed no difference (p> 0.05).
The neuron counts revealed a significant decline in the total neuronal population of groups G50, G100 and G5000 (Figure 1A), while the nitrergic subpopulation (NADPH-diaphorase positive or NADPH-d+) showed an increase in G10, G100, G500 and G5000 (p<0.05) (Figure 1B).
Number of myenteric neurons of the colon of rats infected with different Toxoplasma gondii inoculum loads, expressed in neurons/microscopic field by the Giemsa (A) and NADPH-diaphorase techniques (B). ANOVA followed by Dunnett's multiple comparison post hoc test.
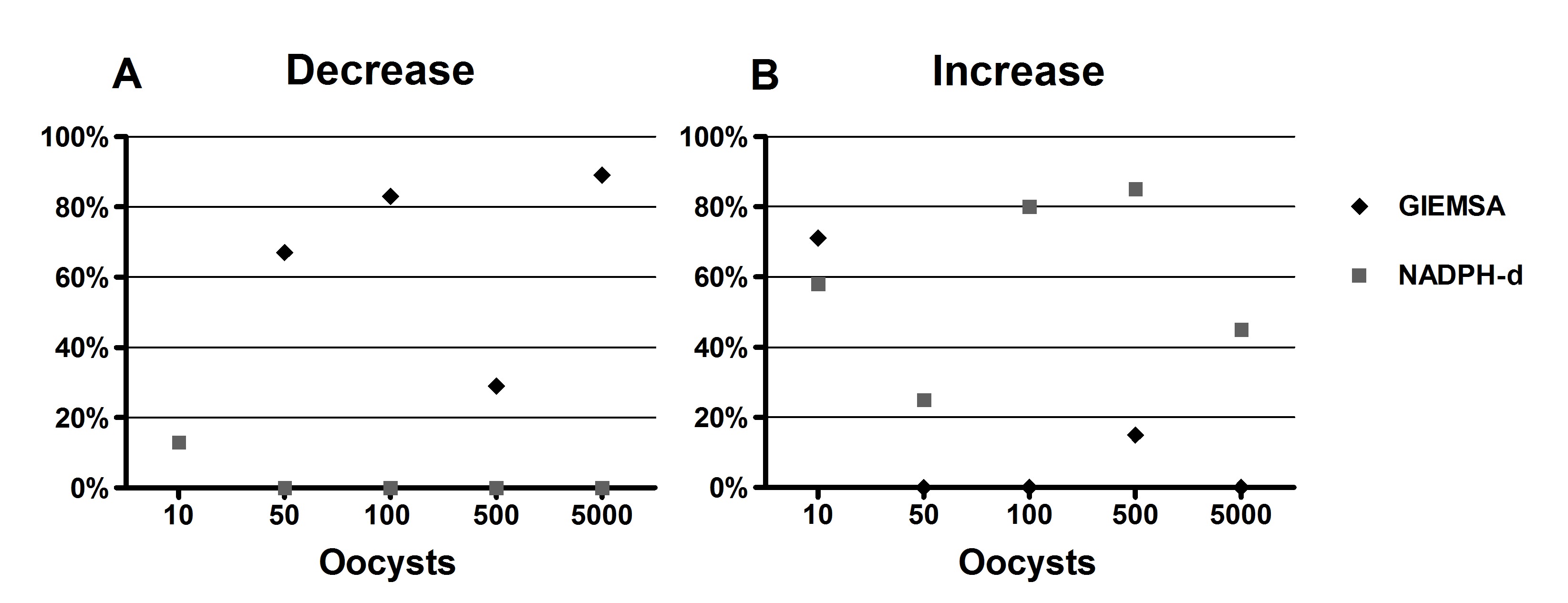
The Giemsa technique (Figure 2A-C) revealed a decline in the number of neurons in the myenteric plexus in the colon of rats infected with 50, 100 or 5000 oocysts. A comparison of the number of myenteric neurons of each animal in the infected groups and the average number presented by the CG and its confidence interval by both techniques indicated that 83% of the animals in G100 and 86% in G5000 presented neuronal loss. These data are consistent with the statistical results, which showed a significant decrease in the average number of neurons. However, all the infected groups had animals that presented this result, particularly G50, in which 67% of the animals showed decreasing numbers. Unlike what was observed in the total neuronal population, the NADPH-d+ technique (Figure 2D-F) revealed a quantitative increase in the nitrergic subpopulation. An individual analysis indicated a tendency for the number of NADPH-d+ neurons to increase, particularly in 86% of the animals in G500 and 80% of those in G100. Figure 3 depicts individual variations in the animals in each group that presented an increase or decrease in the number of neurons.
Photomicrographs showing different neuronal densities by the Giemsa (A=CG, B=G100 and C=G5000) and NADPH-diaphorase (D=CG, E=G500 and F=G5000) techniques. 400X.
Percentage of animals in groups 10, 50, 100, 500 and 500 oocysts0 that showed a decrease (A) and an increase (B) in neuronal counts compared to Control group, by the Giemsa and NADPH-diaphorase techniques.
Discussion
Despite the established infection, changes in the population of enteric neurons were observed mainly in larger inoculum loads, and reveals the total population of neurons and that the average area of the colon in the infected groups remained unchanged, neuronal loss related to T. gondii infection probably occurred.. This result may be due to the action of chemical mediators that are associated with the inflammatory response triggered by T. gondii, including nitric oxide (NO) and IFN-γ, which participate in the mechanism of apoptotic induction and are linked to morphological and quantitative changes of enteric neurons in protozoal infections (ARANTES et al., 2004Arantes RM, Marche HH, Bahia MT, Cunha FQ, Rossi MA, Silva JS. Interferon-γ-induced nitric oxide causes intrinsic intestinal denervation in -infected mice. Trypanosoma cruziAm J Pathol 2004; 164(4): 1361-1368. PMid:15039223. http://dx.doi.org/10.1016/S0002-9440(10)63222-1.
http://dx.doi.org/10.1016/S0002-9440(10)...
; NISHIKAWA et al., 2007Nishikawa Y, Kawase O, Vielemeyer O, Suzuki H, Joiner KA, Xuan X, et al. infection induces apoptosis in noninfected macrophages: role of nitric oxide and other soluble factors. Toxoplasma gondiiParasite Immunol 2007; 29(7): 375-385. PMid:17576367. http://dx.doi.org/10.1111/j.1365-3024.2007.00956.x.
http://dx.doi.org/10.1111/j.1365-3024.20...
). On the other hand, other studies of the colon of rats infected with T. gondii found no changes in the total number of neurons of the myenteric plexus (SOARES et al., 2009Soares J, Moreira NM, Silva AV, Sant’Ana DMG, Araújo EJA. Infecção crônica por Toxoplasma gondii induzindo hipertrofia de neurônios do plexo mientérico do cólon descendente de Rattus norvegicus.Rev Bras Parasitol Vet 2009; 18(2): 57-60. PMid:19602320. http://dx.doi.org/10.4322/rbpv.01802013.
http://dx.doi.org/10.4322/rbpv.01802013...
; SUGAUARA et al., 2008Sugauara EYY, Sant’Ana DMG, Almeida EC, Reis AB, Silva AV, Araújo EJA. Alterations of the myenteric plexus of the ileum and the descending colon caused by (genotype III). Toxoplasma gondiiArq Neuropsiquiatr 2008; 66(3A): 516-523. PMid:18813711. http://dx.doi.org/10.1590/S0004-282X2008000400015.
http://dx.doi.org/10.1590/S0004-282X2008...
). This divergence in findings is explained by differences in the experimental protocol, such as the evolutionary form of the inoculated parasite and the genotype of the strain. A study of the small intestine in which a similar experimental protocol was used revealed neuronal loss with inoculum loads of 10, 100, 500 and 5000 oocysts, demonstrating a greater depletion effect of the infection on neurons in the initial part of the intestines (VICENTINO-VIEIRA et al., 2015Vicentino-Vieira SL, Melo GAN, Góis MB, Moreira NM, Pereira LGA, Araújo EJA, et al. Oral dependent-dose toxoplasmic infection model induced by oocysts in rats: Myenteric plexus and jejunal wall changes. Exp Parasitol 2015; 156: 12-18. PMid:26008610. http://dx.doi.org/10.1016/j.exppara.2015.05.007.
http://dx.doi.org/10.1016/j.exppara.2015...
). This can be explained by the natural presence of a larger number of intraepithelial lymphocytes in the small intestine, resulting in a more intense immune response with greater release of cytokines and other inflammatory mediators, and hence, greater tissue damage (BEAGLEY et al., 1995Beagley KW, Fujihashi K, Lagoo AS, Lagoo-Deenadaylan S, Black CA, Murray AM, et al. Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J Immunol 1995; 154(11): 5611-5619. PMid:7751614.). The release of T. gondii sporozoites also reportedly occurs in the small intestine (FERGUSON & DUBREMETZ, 2007Ferguson DJP, Dubremetz JF. The ultrastructure of . In: Toxoplasma gondiiWeiss L, Kim K. Toxoplasma gondii: the model apicomplexan: perspectives and methods. Rio de Janeiro: Elsevier; 2007. p. 19-48.), which appears to trigger a more intense inflammatory response in this portion of the GIT, also suggesting that neuronal loss seems not to be caused by direct action of the parasite.
Due to the complexity of the biological systems, individuals within the same group respond differently, despite all the care taken to control the variables. It should be noted that the neuronal assessment involved 120 different and random microscopic fields and that the quantitative analysis was blind.
These analyses reinforce the belief that nitrergic neurons are more resistant to apoptosis (COWEN et al., 2000Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut 2000; 47(5): 653-660. PMid:11034581. http://dx.doi.org/10.1136/gut.47.5.653.
http://dx.doi.org/10.1136/gut.47.5.653...
; PHILLIPS et al., 2003Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci 2003; 106(2): 69-83. PMid:12878075. http://dx.doi.org/10.1016/S1566-0702(03)00072-9.
http://dx.doi.org/10.1016/S1566-0702(03)...
), and that after contact with T. gondii, a portion of the total surviving neuronal population begins to express the enzyme NADPH-diaphorase, and therefore NOS-I, in addition to those they already expressed under normal conditions. This phenomenon may indicate a relationship between the immune response against the parasite and the production of nitric oxide in the myenteric plexus. This is corroborated by similar studies of the jejunum of these animals (VICENTINO-VIEIRA et al., 2015Vicentino-Vieira SL, Melo GAN, Góis MB, Moreira NM, Pereira LGA, Araújo EJA, et al. Oral dependent-dose toxoplasmic infection model induced by oocysts in rats: Myenteric plexus and jejunal wall changes. Exp Parasitol 2015; 156: 12-18. PMid:26008610. http://dx.doi.org/10.1016/j.exppara.2015.05.007.
http://dx.doi.org/10.1016/j.exppara.2015...
) and that of pigs (ODORIZZI et al., 2010Odorizzi L, Moreira NM, Gonçalves GF, Silva AV, Sant’Ana DMG, Araújo EJA. Quantitative and morphometric changes of subpopulations of myenteric neurons in swines with toxoplasmosis. Auton Neurosci 2010; 155(1-2): 68-72. PMid:20167543. http://dx.doi.org/10.1016/j.autneu.2010.01.012.
http://dx.doi.org/10.1016/j.autneu.2010....
), in which a genotype III strain was used. However, no studies were found about the effects of toxoplasmosis infection on the NADPH-d+ neurons specific to the colon.
The muscle layers of the intestine are densely innervated by the nerve fibers of the myenteric plexus. The neurons in this plexus can be divided into two different functional types: inhibitory, whose transmission, called non-adrenergic non-cholinergic (NANC), is identified by the concomitant presence of the enzyme NOS and vasoactive intestinal peptide (VIP), and excitatory motor neurons, which are revealed by immunoreactivity to tachykinins and to the vesicular acetylcholine transporter, which are considered cholinergic neurons (DE GIORGIO et al., 1994De Giorgio R, Parodi JE, Brecha NC, Brunicardi FC, Becker JM, Go VL, et al. Nitric oxide producing neurons in the monkey and human digestive system. J Comp Neurol 1994; 342(4): 619-627. PMid:8040367. http://dx.doi.org/10.1002/cne.903420409.
http://dx.doi.org/10.1002/cne.903420409...
; FERRI et al., 1983Ferri GL, Adrian TE, Ghatei MA, O’Shaughnessy DJ, Probert L, Lee YC, et al. Tissue localization and relative distribution of regulatory peptides in separated layers from the human bowel. Gastroenterology 1983; 84(4): 777-786. PMid:6186565.; LLEWELLYN SMITH et al., 1984Llewellyn-Smith IJ, Furness JB, Murphy R, O’Brien PE, Costa M. Substance P-containing nerves in the human small intestine. Distribution, ultrastructure, and characterization of the immunoreactive peptide. Gastroenterology 1984; 86(3): 421-435. PMid:6198237.). Inhibitory and excitatory neurons innervate the muscle with different fibers (WATTCHOW et al., 1988Wattchow DA, Furness JB, Costa M. Distribution and coexistence of peptides in nerve fibers of the external muscle of the human gastrointestinal tract. Gastroenterology 1988; 95(1): 32-41. PMid:2453391. http://dx.doi.org/10.1016/0016-5085(88)90287-9.
http://dx.doi.org/10.1016/0016-5085(88)9...
).
The increase in the NOS+ neuron population, occurring simultaneously with the reduction of the total population, leads us to suppose that the myenteric neuronal depletion caused by toxoplasmosis infection was concentrated in the cholinergic neurons, causing a downward trend in the contractility of the fibers of the intestinal smooth muscle, a phenomenon that may lead to motility disorders. Less contractile muscle fibers lead to slower intestinal flow and constipation, although this sign was not detected in the rats of the infected groups. Because the ME49 strain is not highly virulent, the tissue damage and possible neuronal loss may have been insufficient to trigger noticeable changes during the infection period under study.
In this study, we concluded that the depletion effect of the genotype II strain of T. gondii on the myenteric neurons of the rat colon is significantly achieved through oral inoculation with 5,000 oocysts, and that this effect seems to occur mainly on the cholinergic population of these cells. An increase in the NADPH-d+ subpopulation was also found in response to the inoculation of 10, 100, 500 or 5,000 oocysts. This experimental model proved to be suitable for studies of the chronic phase of T. gondii with an infection time of 30 days.
To help elucidate the phenomena found in this work, additional studies are needed to assess changes in the intestinal tract. Further research is also needed to assess not only quantitative effects but also atrophy and hypertrophy of the myenteric neurons of the colon in infections with larger numbers of oocysts, employing techniques that reveal both nitrergic and cholinergic subpopulations. Studies aimed at clarifying the role of cellular immune response and its mediators. i.e., intraepithelial lymphocytes, macrophages, IFN-γ, NO, cytokines, etc., are already ongoing and may help shed light on the effects of T. gondii infection in the myenteric plexus and in the other structures of the intestinal wall.
References
- Arantes RM, Marche HH, Bahia MT, Cunha FQ, Rossi MA, Silva JS. Interferon-γ-induced nitric oxide causes intrinsic intestinal denervation in -infected mice. Trypanosoma cruziAm J Pathol 2004; 164(4): 1361-1368. PMid:15039223. http://dx.doi.org/10.1016/S0002-9440(10)63222-1
» http://dx.doi.org/10.1016/S0002-9440(10)63222-1 - Araújo EJA, Zaniolo LM, Vicentino SL, Góis MB, Zanoni JN, Silva AV, et al. causes death and plastic alteration in the jejunal myenteric plexus. Toxoplasma gondiiWorld J Gastroenterol 2015; 21(16): 4829-4839. PMid:25944996. http://dx.doi.org/10.3748/wjg.v21.i16.4829
» http://dx.doi.org/10.3748/wjg.v21.i16.4829 - Barbosa AJA. Técnica histológica para gânglios nervosos intramurais em preparados espessos. Rev Bras Pesqui Med Biol 1978; 11(2-3): 95-97. PMid:80017.
- Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012; 380(9853): 1590-1605. PMid:22914295. http://dx.doi.org/10.1016/S0140-6736(12)60026-9
» http://dx.doi.org/10.1016/S0140-6736(12)60026-9 - Beagley KW, Fujihashi K, Lagoo AS, Lagoo-Deenadaylan S, Black CA, Murray AM, et al. Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J Immunol 1995; 154(11): 5611-5619. PMid:7751614.
- Bonapaz RS, Hermes-Uliana C, Santos FN, Silva AV, Araújo EJA, Sant’Ana DMG. Effects of infection with Toxoplasma gondii oocysts on the intestinal wall and myenteric plexus of chicken (). Gallus gallusPesqui Vet Bras 2010; 30(9): 787-792. http://dx.doi.org/10.1590/S0100-736X2010000900013
» http://dx.doi.org/10.1590/S0100-736X2010000900013 - Cowen T, Johnson RJ, Soubeyre V, Santer RM. Restricted diet rescues rat enteric motor neurones from age related cell death. Gut 2000; 47(5): 653-660. PMid:11034581. http://dx.doi.org/10.1136/gut.47.5.653
» http://dx.doi.org/10.1136/gut.47.5.653 - De Giorgio R, Parodi JE, Brecha NC, Brunicardi FC, Becker JM, Go VL, et al. Nitric oxide producing neurons in the monkey and human digestive system. J Comp Neurol 1994; 342(4): 619-627. PMid:8040367. http://dx.doi.org/10.1002/cne.903420409
» http://dx.doi.org/10.1002/cne.903420409 - Desmonts G, Remington JS. Direct agglutination test for diagnosis of infection: method of increasing sensitivity and specificity. ToxoplasmaJ Clin Microbiol 1980; 11(6): 562-568. PMid:7000807.
- Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev 1998; 11(2): 267-299. PMid:9564564.
- Dubey JP. The history and life cycle of . In: Toxoplasma gondiiWeiss L, Kim K. Toxoplasma gondii: the model apicomplexan: perspectives and methods. Rio de Janeiro: Elsevier; 2007. p. 1-17.
- Dusse LMS, Vieira LM, Carvalho MG. Revisão sobre óxido nítrico. J Bras Patol Med Lab 2003; 39(4): 343-350. http://dx.doi.org/10.1590/S1676-24442003000400012
» http://dx.doi.org/10.1590/S1676-24442003000400012 - Ferezin RI, Bertolini DA, Demarchi IG. Prevalência de sorologia positiva para HIV, hepatite B, toxoplasmose e rubéola em gestantes do noroeste paranaense. Rev Bras Ginecol Obstet 2013; 35(2): 66-70. PMid:23412005. http://dx.doi.org/10.1590/S0100-72032013000200005
» http://dx.doi.org/10.1590/S0100-72032013000200005 - Ferguson DJP, Dubremetz JF. The ultrastructure of . In: Toxoplasma gondiiWeiss L, Kim K. Toxoplasma gondii: the model apicomplexan: perspectives and methods. Rio de Janeiro: Elsevier; 2007. p. 19-48.
- Ferri GL, Adrian TE, Ghatei MA, O’Shaughnessy DJ, Probert L, Lee YC, et al. Tissue localization and relative distribution of regulatory peptides in separated layers from the human bowel. Gastroenterology 1983; 84(4): 777-786. PMid:6186565.
- Freyre A, Falcón J, Correa O, Mendez J, González M, Venzal JM. Residual infection of 15 toxoplasma strains in the brain of rats fed cysts. Parasitol Res 2001; 87(11): 915-918. PMid:11728015.
- Furness JB. The enteric nervous system. Malden: Blackwell; 2006.
- Góis MB, Hermes-Uliana C, Zago MCB, Zanoni JN, Silva AV, Miranda MH No, et al. Chronic infection with induces death of submucosal enteric neurons and damage in the colonic mucosa of rats. Toxoplasma gondiiExp Parasitol 2016a; 164: 56-63. PMid:26902605. http://dx.doi.org/10.1016/j.exppara.2016.02.009
» http://dx.doi.org/10.1016/j.exppara.2016.02.009 - Góis MB, Hermes-Uliana C, Paltanin A, Pontes W, Araújo EJA, Miranda MH No, et al. Morphoquantitative study of submucosal plexus by different neuronal evidentiation histochemical techniques. Rattus norvegicusInt J Morphol. 2016b; 34(4): 1487-1493.
- Hermes-Uliana C, Pereira-Severi LS, Luerdes RB, Franco CLM, Silva AV, Araújo EJA, et al. Chronic infection with causes myenteric neuroplasticity of the jejunum in rats. Toxoplasma gondiiAuton Neurosci 2011; 160(1-2): 3-8. PMid:20932812. http://dx.doi.org/10.1016/j.autneu.2010.09.003
» http://dx.doi.org/10.1016/j.autneu.2010.09.003 - Howe DK, Sibley LD. comprises three clonal lineages: correlation of parasite genotype with human disease. Toxoplasma gondiiJ Infect Dis 1995; 172(6): 1561-1566. PMid:7594717. http://dx.doi.org/10.1093/infdis/172.6.1561
» http://dx.doi.org/10.1093/infdis/172.6.1561 - Iyengar R, Stuehr DJ, Marletta MA. Macrophage synthesis of nitrite, nitrate and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci USA 1987; 84(18): 6369-6373. PMid:2819872. http://dx.doi.org/10.1073/pnas.84.18.6369
» http://dx.doi.org/10.1073/pnas.84.18.6369 - Liesenfeld O. Oral infection of C57BL/6 mice with Toxoplasma gondii: a new model of inflammatory bowel disease? J Infect Dis 2002;185(Suppl 1): 96-101. PMid:11865446. http://dx.doi.org/10.1086/338006
» http://dx.doi.org/10.1086/338006 - Llewellyn-Smith IJ, Furness JB, Murphy R, O’Brien PE, Costa M. Substance P-containing nerves in the human small intestine. Distribution, ultrastructure, and characterization of the immunoreactive peptide. Gastroenterology 1984; 86(3): 421-435. PMid:6198237.
- Lunde MN, Jacobs L. Antigenic differences between endozoites and cystozoites of Toxoplasma gondii.J Parasitol 1983; 69(5): 806-808. PMid:6200590. http://dx.doi.org/10.2307/3281034
» http://dx.doi.org/10.2307/3281034 - Maubon D, Ajzenberg D, Brenier-Pinchart MP, Dardé ML, Pelloux H. What are the respective host and parasite contributions to toxoplasmosis? Trends Parasitol 2008; 24(7): 299-303. PMid:18514029. http://dx.doi.org/10.1016/j.pt.2008.03.012
» http://dx.doi.org/10.1016/j.pt.2008.03.012 - Meyers S, Janowitz HD. The “natural history” of ulcerative colitis: an analysis of the placebo response. J Clin Gastroenterol 1989; 11(1): 33-37. PMid:2646359. http://dx.doi.org/10.1097/00004836-198902000-00008
» http://dx.doi.org/10.1097/00004836-198902000-00008 - Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004; 363(9425): 1965-1976. PMid:15194258. http://dx.doi.org/10.1016/S0140-6736(04)16412-X
» http://dx.doi.org/10.1016/S0140-6736(04)16412-X - Nishikawa Y, Kawase O, Vielemeyer O, Suzuki H, Joiner KA, Xuan X, et al. infection induces apoptosis in noninfected macrophages: role of nitric oxide and other soluble factors. Toxoplasma gondiiParasite Immunol 2007; 29(7): 375-385. PMid:17576367. http://dx.doi.org/10.1111/j.1365-3024.2007.00956.x
» http://dx.doi.org/10.1111/j.1365-3024.2007.00956.x - Odorizzi L, Moreira NM, Gonçalves GF, Silva AV, Sant’Ana DMG, Araújo EJA. Quantitative and morphometric changes of subpopulations of myenteric neurons in swines with toxoplasmosis. Auton Neurosci 2010; 155(1-2): 68-72. PMid:20167543. http://dx.doi.org/10.1016/j.autneu.2010.01.012
» http://dx.doi.org/10.1016/j.autneu.2010.01.012 - Pereira LS, Silva AV, Araújo EJA, Sant’Ana DMG. Hypertrophy of NADH-diaphorase positive myenteric neurons in rat jejunum after acute infection caused by Toxoplasma gondii.J Venom Anim Toxins Incl Trop Dis 2010; 16(2): 298-310.
- Phillips RJ, Kieffer EJ, Powley TL. Aging of the myenteric plexus: neuronal loss is specific to cholinergic neurons. Auton Neurosci 2003; 106(2): 69-83. PMid:12878075. http://dx.doi.org/10.1016/S1566-0702(03)00072-9
» http://dx.doi.org/10.1016/S1566-0702(03)00072-9 - Reiche EMV, Morimoto HK, Farias GN, Hisatsugu KR, Geller L, Gomes ACLF, et al. Prevalência de tripanossomíase americana, sífilis, toxoplasmose, rubéola, hepatite B, hepatite C e da infecção pelo vírus da imunodeficiência humana, avaliada por intermédio de testes sorológicos, em gestantes atendidas no período de 1996 a 1998 no Hospital Universitário Regional Norte do Paraná (Universidade Estadual de Londrina, Paraná, Brasil). Rev Soc Bras Med Trop 2000; 33(6): 519-527. PMid:11175581. http://dx.doi.org/10.1590/S0037-86822000000600002
» http://dx.doi.org/10.1590/S0037-86822000000600002 - Sartori AL, Minamisava R, Avelino MM, Martins CA. Triagem pré-natal para toxoplasmose e fatores associados à soropositividade de gestantes em Goiânia, Goiás. Rev Bras Ginecol Obstet 2011; 33(2): 93-98. PMid:21779652. http://dx.doi.org/10.1590/S0100-72032011000200007
» http://dx.doi.org/10.1590/S0100-72032011000200007 - Scherer-Singler U, Vincent SR, Kimura H, McGeer EG. Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods 1983; 9(3): 229-234. PMid:6363828. http://dx.doi.org/10.1016/0165-0270(83)90085-7
» http://dx.doi.org/10.1016/0165-0270(83)90085-7 - Schreiner M, Liesenfeld O. Small intestinal inflammation following oral infection with Toxoplasma gondii does not occur exclusively in C57BL/6 mice: review of 70 reports from the literature. Mem Inst Oswaldo Cruz 2009; 104(2): 221-233. PMid:19430647. http://dx.doi.org/10.1590/S0074-02762009000200015
» http://dx.doi.org/10.1590/S0074-02762009000200015 - Silva AV, Pezerico SB, Lima VY, d’Arc Moretti L, Pinheiro JP, Tanaka EM, et al. Genotyping of strains isolated from dogs with neurological signs. Toxoplasma gondiiVet Parasitol 2005; 127(1): 23-27. PMid:15619371. http://dx.doi.org/10.1016/j.vetpar.2004.08.020
» http://dx.doi.org/10.1016/j.vetpar.2004.08.020 - Soares J, Moreira NM, Silva AV, Sant’Ana DMG, Araújo EJA. Infecção crônica por Toxoplasma gondii induzindo hipertrofia de neurônios do plexo mientérico do cólon descendente de Rattus norvegicus.Rev Bras Parasitol Vet 2009; 18(2): 57-60. PMid:19602320. http://dx.doi.org/10.4322/rbpv.01802013
» http://dx.doi.org/10.4322/rbpv.01802013 - Sugauara EYY, Sant’Ana DMG, Almeida EC, Reis AB, Silva AV, Araújo EJA. Alterations of the myenteric plexus of the ileum and the descending colon caused by (genotype III). Toxoplasma gondiiArq Neuropsiquiatr 2008; 66(3A): 516-523. PMid:18813711. http://dx.doi.org/10.1590/S0004-282X2008000400015
» http://dx.doi.org/10.1590/S0004-282X2008000400015 - Trevizan AR, Vicentino-Vieira SL, Silva Watanabe P, Góis MB, de Melo GAN, Garcia JL, et al. Kinetics of acute infection with and histopathological changes in the duodenum of rats. Toxoplasma gondiiExp Parasitol 2016; 165: 22-29. PMid:26993084. http://dx.doi.org/10.1016/j.exppara.2016.03.015
» http://dx.doi.org/10.1016/j.exppara.2016.03.015 - Vicentino-Vieira SL, Melo GAN, Góis MB, Moreira NM, Pereira LGA, Araújo EJA, et al. Oral dependent-dose toxoplasmic infection model induced by oocysts in rats: Myenteric plexus and jejunal wall changes. Exp Parasitol 2015; 156: 12-18. PMid:26008610. http://dx.doi.org/10.1016/j.exppara.2015.05.007
» http://dx.doi.org/10.1016/j.exppara.2015.05.007 - Vivas LAM, Jamel N, Refinetti RA, Silva LF, Rodrigues LV, Silva PC, et al. Anesthetic experimental device for small animal. Acta Cir Bras 2007; 22(3): 229-233. PMid:17546297. http://dx.doi.org/10.1590/S0102-86502007000300012
» http://dx.doi.org/10.1590/S0102-86502007000300012 - Wattchow DA, Furness JB, Costa M. Distribution and coexistence of peptides in nerve fibers of the external muscle of the human gastrointestinal tract. Gastroenterology 1988; 95(1): 32-41. PMid:2453391. http://dx.doi.org/10.1016/0016-5085(88)90287-9
» http://dx.doi.org/10.1016/0016-5085(88)90287-9 - Weiss L, Kim K. Toxoplasma gondii: the model apicomplexan: perspectives and methods. Rio de Janeiro: Elsevier; 2007.
- Zar JH. Biostatistical analysis. 4th. ed. New Jersey: Prentice Hall; 1999.
Publication Dates
-
Publication in this collection
Jan-Mar 2017
History
-
Received
14 Oct 2016 -
Accepted
17 Jan 2017