ABSTRACT
Background
Improved targeted therapies for rheumatic diseases were developed recently resulting in a better prognosis for affected patients. Nowadays, patients are living longer and with improved quality of life, including fertility potential. These patients are affected by impaired reproductive function and the causes are often multifactorial related to particularities of each disease. This review highlights how rheumatic diseases and their management affect testicular function and male fertility.
Materials and Methods
A systematic review of literature of all published data after 1970 was conducted. Data was collected about fertility abnormalities in male patients with systemic lupus erythematosus, rheumatoid arthritis, dermatomyositis, ankylosing spondylitis, Behçet disease and gout. Two independent researchers carried out the search in online databases.
Results
A total of 19 articles were included addressing the following diseases: 7 systemic lupus erythematosus, 6 Behçet disease, 4 ankylosing spondylitis, 2 rheumatoid arthritis, 2 dermatomyositis and one gout. Systemic lupus erythematosus clearly affects gonadal function impairing spermatogenesis mainly due to antisperm antibodies and cyclophosphamide therapy. Behçet disease, gout and ankylosing spondylitis patients, including those under anti-TNF therapy in the latter disease, do not seem to have reduced fertility whereas in dermatomyositis, the fertility potential is hampered by disease activity and by alkylating agents. Data regarding rheumatoid arthritis is scarce, gonadal dysfunction observed as consequence of disease activity and antisperm antibodies.
Conclusions
Reduced fertility potential is not uncommon. Its frequency and severity vary among the different rheumatic diseases. Permanent infertility is rare and often associated with alkylating agent therapy.
Rheumatic Diseases; Fertility; Infertility, Male
INTRODUCTION
There are 1.3 million adults affected by rheumatoid arthritis (RA) and up to 322.000 by systemic lupus erythematosus (SLE) in United States (11. Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008; 58:15-25.). Improved targeted therapies for rheumatic diseases have been developed recently resulting in better prognosis. In this context health-related quality of life became a major concern, including reproductive issues (22. Østensen M. New insights into sexual functioning and fertility in rheumatic diseases. Best Pract Res Clin Rheumatol. 2004; 18:219-32.).
Decreased fertility potential is not unusual among patients of both genders with rheumatic diseases, particularly in women (33. Clowse ME, Chakravarty E, Costenbader KH, Chambers C, Michaud K. Effects of infertility, pregnancy loss, and patient concerns on family size of women with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2012; 64:668-74., 44. Gupta R, Deepanjali S, Kumar A, Dadhwal V, Agarwal SK, Pandey RM, et al. A comparative study of pregnancy outcomes and menstrual irregularities in northern Indian patients with systemic lupus erythematosus and rheumatoid arthritis. Rheumatol Int. 2010; 30:1581-5.) with many articles addressing in RA, SLE, ankylosing spondylitis (AS), dermatomyositis (DM), Behçet disease (BD) and gout (55. Silva CA, Bonfa E, Østensen M. Maintenance of fertility in patients with rheumatic diseases needing antiinflammatory and immunosuppressive drugs.Arthritis Care Res (Hoboken). 2010; 62:1682-90.
6. Martínez Lopez JA, Loza E, Carmona L. Systematic review on the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy, and breastfeeding). Clin Exp Rheumatol. 2009; 27:678-84.
7. Paschou S, Voulgari PV, Vrabie IG, Saougou IG, Drosos AA. Fertility and reproduction in male patients with ankylosing spondylitis treated with infliximab. J Rheumatol. 2009; 36:351-4.-88. Hersh A, von Scheven E, Yelin E. Adult outcomes of childhood-onset rheumatic diseases. Nat Rev Rheumatol. 2011; 7:290-5.). Drug treatment is probably the main factor for gonadal dysfunction (99. Freire EA, Nepomuceno JD, Maia ID, Ciconelli RM. Doenças reumáticas e infertilidade masculina. Revista Brasileira de Reumatologia. 2006;46:12-20.). Some drugs can cause reversible infertility, such as nonsteroidal antiinflammatory drugs in women and sulfasalazine/methotrexate in men whereas irreversible infertility is occasionally observed after treatment with alkylating agents (cyclophosphamide-CYC and chlorambucil) in both genders (1010. Dooley MA, Nair R. Therapy Insight: preserving fertility in cyclophosphamide-treated patients with rheumatic disease. Nat Clin Pract Rheumatol. 2008; 4:250-7.). When fertility is an issue, alkylating agents should be used at lowest possible dose and alternative therapies (such as azathioprine or mycophenolate mofetil) must be considered.
The reproduction potential of these male patients is impaired by the disease directly in the testicular tissue or by immunosuppressive therapy (1111. Miller MH, Urowitz MB, Gladman DD, Killinger DW. Systemic lupus erythematosus in males. Medicine (Baltimore). 1983; 62:327-34.). The evaluation of male subjects should rely on careful medical history, complete physical examination, semen analysis and sexual hormone profile.
The objective of this systematic review of the literature on rheumatic disease male fertility potential is to provide a better understanding to urologists, andrologists, infertility specialists and rheumatologists of the underlying contributing factors involved, as well as discuss how fertility potential is endangered by diseases management.
SEARCH STRATEGY AND SELECTION CRITERIA
It was conducted a computerized search of English and non-English language articles published after 1970 listed in the electronic databases of SCOPUS, PUBMED/MEDLINE and Cochrane Library. Two independent researchers (MC, BT) conducted the search during May-July 2014. The following terms were used: ‘systemic lupus erythematosus’, ‘ankylosing spondylitis’, ‘dermatomyositis’, ‘rheumatoid arthritis’, ‘Behçet disease’, ‘gout’, ‘male infertility’, ‘pregnancy rate’, ‘sperm’, ‘semen’, ‘spermatozoa’, ‘sperm quality’ and ‘rheumatic disease’. The search was performed in English language but articles yielded in other languages were not excluded. The authors graded the abstract of each study identified by the search to determine eligibility. If these criteria remained unclear from the abstract, the full article was retrieved for clarification.
Data extraction was carried out by the investigators using a standardized data collection form with subsequent discussion with all authors. Peer-reviewed observational controlled and non-controlled studies (case–control and cohort studies) were selected. All studies were referral centre-, hospital-or population-based studies. The data collected in the selected articles were all related to fertility abnormalities in male patients with SLE, RA, DM, AS, BD and gout. We excluded articles that were case reports and those that did not evaluated male patients.
RESULTS
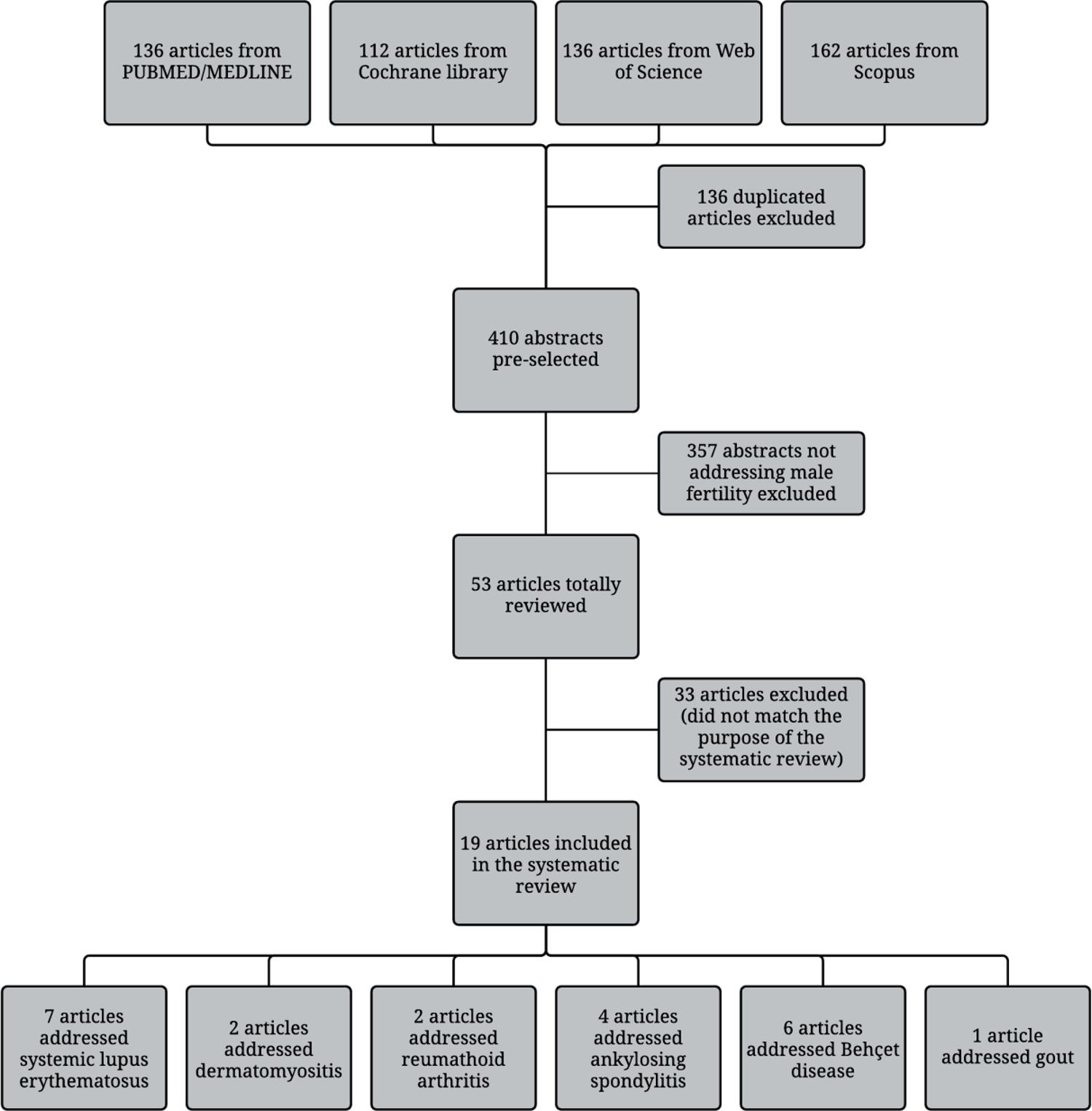
The article flow through the selection phase is summarized in Figure-1. An initial search of online databases yielded 136 publications from PUBMED/MEDLINE, 112 reviews from Cochrane Library, 136 from Web of Science, and 162 from Scopus. After excluding duplicated publications and applying exclusion criteria, 19 relevant articles were included with the following diseases: 7 SLE, 2 DM, 2 RA, 4 AS, 6 BD and one with gout. There was one article evaluating simultaneously two diseases and another addressing three (Figure-1).
Systemic lupus erythematosus
Publications selected focused on four aspects of male fertility in SLE: gonadal dysfunction, testicular alterations induced by immunosuppressive treatment, presence of anti-sperm antibody and genetic abnormalities (Table-1).
A global gonadal function evaluation was performed by our Group (1212. Soares PM, Borba EF, Bonfa E, Hallak J, Corrêa AL, Silva CA. Gonad evaluation in male systemic lupus erythematosus. Arthritis Rheum. 2007; 56:2352-61.) assessing sex hormone profile, semen analysis and antisperm analysis (ASA). Thirty-five patients compared to paired controls had lower testicular volumes, total sperm count and total motile sperm count associated with CYC use.
We investigated Sertoli cell function analyzing inhibin B levels and semen abnormalities in SLE patients. Lower inhibin B level was correlated with diminished sperm count, concentration and total motility count and with elevated FSH and LH levels (1313. Suehiro RM, Borba EF, Bonfa E, Okay TS, Cocuzza M, Soares PM, et al. Testicular Sertoli cell function in male systemic lupus erythematosus. Rheumatology (Oxford). 2008; 47:1692-7.). In addition, it was observed that 20% of SLE patients had erectile dysfunction, 36% of testicles were below the normal volume range and 48% had semen analysis abnormalities associated with CYC therapy (1414. Silva CA, Bonfá E, Borba EF, Braga AP, Soares PM, Moraes AJ, et al. Saúde reprodutiva em homens com lúpus eritematoso sistêmico. Revista Brasileira de Reumatologia. 2009;49:207-22.). The same gonadotoxic effect of CYC was also reported in four patients with juvenile SLE (1515. Silva CA, Hallak J, Pasqualotto FF, Barba MF, Saito MI, Kiss MH. Gonadal function in male adolescents and young males with juvenile onset systemic lúpus erythematosus. J Rheumatol. 2002; 29:2000-5.).
Serum IgG ASA targeting the sperm head and/or midpiece was reported in 15% and antisperm deoxyribonucleic acid antibodies were found in 42% of SLE patients, indicating that autoimmunity is another contributing factor in these patient’s (1616. D’Cruz OJ, Haas GG Jr, Reichlin M. Autoantibodies to decondensed sperm nuclear deoxyribonucleic acid in patients with antisperm antibodies and systemic lúpus erythematosus detected by immunofluorescence flow cytometry. Fertil Steril. 1994; 62:834-44.). This finding was confirmed in 8 patients evaluated by Shiraishi et al. (1717. Shiraishi Y, Shibahara H, Koriyama J, Hirano Y, Okazaki H, Minota S, et al. Incidence of antisperm antibodies in males with systemic autoimmune diseases. Am J Reprod Immunol. 2009; 61:183-9.).
Recently, Dillon et al. (1818. Dillon SP, Kurien BT, Li S, Bruner GR, Kaufman KM, Harley JB, et al. Sex chromosome aneuploidies among men with systemic lupus erythematosus. J Autoimmun. 2012; 38:J129-34.) evaluated the karyotype of 316 men with SLE and 1201 healthy controls. Aneuploidies were evidenced in 2.5% male SLE patients and none in controls. There was three 47, XXY, three patients with mosaic 46, XY/47, XXY, one had 46, XX/47, XXY mosaicism and another one had 46, XX karyotype.
Dermatomyositis
The two publications addressing DM patient’s fertility are illustrated in Table-2. Moraes et al. (1919. Moraes AJ, Pereira RM, Cocuzza M, Casemiro R, Saito O, Silva CA. Minor sperm abnormalities in young male post-pubertal patients with juvenile dermatomyositis. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al. 2008;41(12):1142-7.) evaluated five patients with juvenile DM and compared with 10 age-matched healthy controls regarding testicular volume, sperm analysis, ASA and sex hormone profile. One patient had used CYC with a cumulative dose of 6.6g and experienced transient azoospermia with normalization after 5 years of medication withdrawal. All DM patients had teratospermia, one had ASA and none had abnormal hormone profile.
A later study investigated 10 adult patients and 10 age-matched healthy controls. DM subjects had lower sperm concentration, lower total motile sperm count and lower normal sperm morphology percentage. Disease activity seemed to be a relevant factor in four patients and CYC in one of them (2020. Moraes AJ, Bonfa E, Cocuzza M, Borges CT, Saito O, Silva CA. Gonad evaluation in male dermatomyositis. A pilot study. Clin Exp Rheumatol. 2010; 28:441-2.).
Rheumatoid arthritis
Two publications assessed fertility on RA patients (Table-3). Gordon et al. (2121. Gordon D, Beastall GH, Thomson JA, Sturrock RD. Androgenic status and sexual function in males with rheumatoid arthritis and ankylosing spondylitis. Q J Med. 1986; 60:671-9.) evaluated 31 patients with RA, 33 with AS and 95 healthy controls. Patients with RA had lower serum testosterone levels and higher FSH and LH levels than controls. Ten patients (33%) admitted periods of erectile dysfunction while 15 (50%) also referred decreased libido when suffering from arthritis. Four patients referred difficulty to conceive, among them, two did not seek medical assistance for infertility. Nineteen males had successfully fathered children and the others were still singles.
In 2008, Shiraishi et al. (1717. Shiraishi Y, Shibahara H, Koriyama J, Hirano Y, Okazaki H, Minota S, et al. Incidence of antisperm antibodies in males with systemic autoimmune diseases. Am J Reprod Immunol. 2009; 61:183-9.) evaluated 32 RA patients and found one with serum ASA. The patient was 74 years old and the disease onset was at the age of 60 years. He had already 2 children before being diagnosed with the disease so the relation between fertility status and the presence of the antibody could not be addressed nor its relation with the disease.
Ankylosing spondylitis
Four publications were selected regarding the AS association with male fertility. The major aspects of each paper are summarized in Table-4. A total of 33 AS patients were evaluated in 1986, reporting four patients with erectile dysfunction and 11 with decreased libido. Only one male with AS had an infertile marriage and did not seek for medical assistance. Thirteen patients were singles and all other had constituted their families without problems (2121. Gordon D, Beastall GH, Thomson JA, Sturrock RD. Androgenic status and sexual function in males with rheumatoid arthritis and ankylosing spondylitis. Q J Med. 1986; 60:671-9.). Varicocele was an additional and frequent finding in AS males (40%), of another cohort, and its impact in male fertility remains to be determined, since only mild sperm abnormalities was observed in these patients (2222. Almeida BP, Saad CG, Souza FH, Moraes JC, Nukumizu LA, Viana VS, et al. Testicular Sertoli cell function in ankylosing spondylitis. Clin Rheumatol. 2013; 32:1075-9.).
Regarding to biological therapy, Paschou et al. (77. Paschou S, Voulgari PV, Vrabie IG, Saougou IG, Drosos AA. Fertility and reproduction in male patients with ankylosing spondylitis treated with infliximab. J Rheumatol. 2009; 36:351-4.), in 2009, assessed AS patients who were treated with infliximab and reviewed their medical records. They found that all of them had successfully fathered at least one children even one patient that also received small doses of methotrexate. Reinforcing this finding, our group evaluated 20 AS patients under TNF blockage and compared to 24 normal controls and showed no difference in sex hormone, inhibin B levels and seminal analysis (2323. Nukumizu LA, Gonçalves Saad C, Ostensen M, Almeida BP, Cocuzza M, Gonçalves C, et al. Gonadal function in male patients with ankylosing spondylitis. Scand J Rheumatol. 2012; 41:476-81.).
Behçet disease
There were six papers addressing BD and its relationship with male fertility. They are summarized in Table-5. An early report brought attention to possible side-effects of colchicine used in BD management with oligospermia in 11 of 136 patient’s (2424. Mizushima Y, Matsumura N, Mori M, Shimizu T, Fukushima B, Mimura Y, et al. Colchicine in Behçet’s disease. Lancet. 1977; 2:1037.). Later, Sarica et al. (2525. Sarica K, Süzer O, Gürler A, Baltaci S, Ozdiler E, Dinçel C. Urological evaluation of Behçet patients and the effect of colchicine on fertility. Eur Urol. 1995; 27:39-42.) evaluated 62 men with BD treated only with colchicine and evidenced oligonecrospermia in 37% and azoospermia in 3%. However, Fukutani et al. (2626. Fukutani K, Ishida H, Shinohara M, Minowada S, Niijima T, Hijikata K, et al. Suppression of spermatogenesis in patients with Behçet’s disease treated with cyclophosphamide and colchicine. Fertil Steril. 1981; 36:76-80.) evaluating 27 BD patients did not observe impact seminal parameters or FSH levels in patients treated with colchicine use alone.
Alkylating agents may induce sperm abnormalities in BD patient’s, as reported in ten men using chlorambucil that had impairment of semen production: 7 with oligospermia and 3 with azoospermia (2727. Tabbara KF. Chlorambucil in Behçet’s disease. A reappraisal. Ophthalmology. 1983; 90:906-8.).
A recent study evaluated fertility outcome of a larger retrospective cohort of BD patient’s compared to a control Group (2828. Uzunaslan D, Saygin C, Hatemi G, Tascilar K, Yazici H. No appreciable decrease in fertility in Behçet’s syndrome. Rheumatology (Oxford). 2014; 53:828-33.). They observed that 23 out of 130 subjects had infertility and the most common etiology was varicocele. In contrast, none of the 14 men with BD assessed for ASA had antisperm antibodies (1717. Shiraishi Y, Shibahara H, Koriyama J, Hirano Y, Okazaki H, Minota S, et al. Incidence of antisperm antibodies in males with systemic autoimmune diseases. Am J Reprod Immunol. 2009; 61:183-9.).
Gout
There is only one report addressing gout and fertility in males (Table-5). In a large study with 540 young patients with gout, fertility status was preserved in patients treated with colchicine and none presented fertility issues during 20 years of follow-up (2929. Yü T. The efficacy of colchicine prophylaxis in articular gout--a reappraisal after 20 years. Semin Arthritis Rheum. 1982; 12:256-64.).
DISCUSSION
SLE is an uncommon disease in men. It affects males in a sex ratio of 1:9 (3030. Wallace DJ, Hahn B, Dubois EL. Dubois’ lupus erythematosus and related syndromes. 8th ed. Philadelphia, PA: Elsevier/Saunders; 2013. xv, pp. 694.), suggesting that sex hormones could modify susceptibility or reduce the expression of SLE (1111. Miller MH, Urowitz MB, Gladman DD, Killinger DW. Systemic lupus erythematosus in males. Medicine (Baltimore). 1983; 62:327-34.). Infertility is an important issue for them nowadays due to better prognosis and quality of life. Six publications reported impaired testicular function and decreased semen production in SLE patient’s and their possible association with disease and treatment (1111. Miller MH, Urowitz MB, Gladman DD, Killinger DW. Systemic lupus erythematosus in males. Medicine (Baltimore). 1983; 62:327-34.
12. Soares PM, Borba EF, Bonfa E, Hallak J, Corrêa AL, Silva CA. Gonad evaluation in male systemic lupus erythematosus. Arthritis Rheum. 2007; 56:2352-61.
13. Suehiro RM, Borba EF, Bonfa E, Okay TS, Cocuzza M, Soares PM, et al. Testicular Sertoli cell function in male systemic lupus erythematosus. Rheumatology (Oxford). 2008; 47:1692-7.-1414. Silva CA, Bonfá E, Borba EF, Braga AP, Soares PM, Moraes AJ, et al. Saúde reprodutiva em homens com lúpus eritematoso sistêmico. Revista Brasileira de Reumatologia. 2009;49:207-22., 3131. Vilarinho ST, Costallat LT. Evaluation of the hypothalamic-pituitary-gonadal axis in males with systemic lupus erythematosus. J Rheumatol. 1998; 25:1097-103., 3232. Mok CC, Lau CS. Profile of sex hormones in male patients with systemic lúpus erythematosus. Lupus. 2000; 9:252-7.). The underlying mechanism for disease induced damage to the testis is not completely elucidated although some authors showed that there is immunopathologic lesion of the testis (and excurrent ducts) occurring through T cell-mediated mechanisms triggered by antigens or pathogens that disrupt testicular immune privilege (3333. Rival C, Guazzone VA, Theas MS, Lustig L. Pathomechanism of autoimune orchitis. Andrologia. 2005; 37:226-7., 3434. Tung KS, Teuscher C. Mechanisms of autoimmune disease in the testis and ovary. Hum Reprod Update. 1995; 1:35-50.). Testicular aggression by alkylating agents has been described since 1972 showing that it impairs the sperm production presenting spermatogenesis abnormalities and Sertoli cell dysfunction (3535. Etteldorf JN, West CD, Pitcock JA, Williams DL. Gonadal function, testicular histology, and meiosis following cyclophosphamide therapy in patients with nephrotic syndrome. J Pediatr. 1976; 88:206-12.
36. Kumar R, Biggart JD, McEvoy J, McGeown MG. Cyclophosphamide and reproductive function. Lancet. 1972; 1:1212-4.-3737. Qureshi MS, Pennington JH, Goldsmith HJ, Cox PE. Cyclophosphamide therapy and sterility. Lancet. 1972; 2:1290-1.).
Although semen analysis is considered the hallmark of male infertility evaluation, standard seminal parameters do not detect abnormalities in up to 20% of subfertile males (3838. Romeo C, Ientile R, Santoro G, Impellizzeri P, Turiaco N, Impalà P, et al. Nitric oxide production is increased in the spermatic veins of adolescents with left idiophatic varicocele. J Pediatr Surg. 2001; 36:389-93.). The routine measurements do not reveal seminal defects at molecular levels that might be induced by reactive oxygen species, which are associated with male infertility (3939. Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005; 95:503-7., 4040. Cocuzza M, Sikka SC, Athayde KS, Agarwal A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: an evidence based analysis. Int Braz J Urol. 2007; 33:603-21.). The 2010 World Health Organization guidelines have reduced the reference limits for seminal parameters and fail to satisfy clinical and statistical standards and pose the risk of misclassifying a subject’s true fertility status (4141. WHO. World Health Organization: WHO Laboratory manual for the examination and processing of human semen - 5th ed. Geneva: WHO Press, 2010., 4242. Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006; 85:629-34.). The introduction of new biomarkers of spermatic function in the clinical practice will allow a more precise evaluation of the real impact of rheumatic disease on male fertility potential. Unfortunately, to date, there is no available information in the literature and these aspects were not covered in the present review.
Autoimmunity also affects fertility by the presence of ASA. Immunologic infertility is characterized by the presence of antibodies against spermatozoa in the serum and/or in the seminal plasma or on the sperm surface (4343. Arap MA, Vicentini FC, Cocuzza M, Hallak J, Athayde K, Lucon AM, et al. Late hormonal levels, semen parameters, and presence of antisperm antibodies in patients treated for testicular torsion. J Androl. 2007; 28:528-32.). The presence of multiple ASA can lead to the immobilization and/or agglutination of spermatozoa, which blocks sperm-egg interaction. They can also prevent implantation or arrest embryo development (4444. Koide SS, Wang L, Kamada M. Antisperm antibodies associated with infertility: properties and encoding genes of target antigens. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY. 2000;224:123-32., 4545. Haas GG Jr. The inhibitory effect of sperm-associated immunoglobulins on cervical mucus penetration. Fertil Steril. 1986;46:334-7. Erratum in: Fertil Steril 1986; 46:1172.). In SLE patient’s ASA have been found in up to 42% of the patient’s. However, the real significance of ASA in infertile men is controversial and currently there is no standardized treatment regimens (4646. Marshburn PB, Kutteh WH. The role of antisperm antibodies in infertility. Fertil Steril. 1994; 61:799-811.). Lastly, aneuploidies are frequent and may also contribute for fertility impairment in SLE patient’s; therefore kariotype should be evaluated to complete the fertility analysis of these patient’s, especially in those with severally compromised spermatogenesis (4747. Fu L, Xiong DK, Ding XP, Li C, Zhang LY, Ding M, et al. Genetic screening for chromosomal abnormalities and Y chromosome microdeletions in Chinese infertile men. J Assist Reprod Genet. 2012; 29:521-7.).
The incidence of DM is 1.5 to 0.7 per 100.000 per person-years and there is a trend to affect more women than males in a 1.9 ratio (4848. See LC, Kuo CF, Chou IJ, Chiou MJ, Yu KH. Sex- and age-specific incidence of autoimmune rheumatic diseases in the Chinese population: a Taiwan population-based study. Semin Arthritis Rheum. 2013; 43:381-6., 4949. Yu KH, See LC, Kuo CF, Chou IJ, Chou MJ. Prevalence and incidence in patients with autoimmune rheumatic diseases: a nationwide population-based study in Taiwan. Arthritis Care Res (Hoboken). 2013; 65:244-50.). The fertility evaluation of this particular Group has limited publications. The most important contributing factors of infertility in male DM are disease activity and CYC use (66. Martínez Lopez JA, Loza E, Carmona L. Systematic review on the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy, and breastfeeding). Clin Exp Rheumatol. 2009; 27:678-84.), generally associated with high doses of this alkylating agent (55. Silva CA, Bonfa E, Østensen M. Maintenance of fertility in patients with rheumatic diseases needing antiinflammatory and immunosuppressive drugs.Arthritis Care Res (Hoboken). 2010; 62:1682-90., 5050. Latta K, von Schnakenburg C, Ehrich JH. A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol. 2001; 16:271-82.).
Several large Scandinavian cohorts and a cohort study in the United States demonstrate that women with RA have smaller families and are slower to conceive compared with other women (5151. Provost M, Eaton JL, Clowse ME. Fertility and infertility in rheumatoid arthritis. Curr Opin Rheumatol. 2014; 26:308-14.). Even though female infertility has been extensively explored in past years, the evaluation of male fertility was overlooked. The few publications available show gonadal impaired function with elevated LH/FSH, patients with difficult to conceive and also a higher incidence of ASA (1717. Shiraishi Y, Shibahara H, Koriyama J, Hirano Y, Okazaki H, Minota S, et al. Incidence of antisperm antibodies in males with systemic autoimmune diseases. Am J Reprod Immunol. 2009; 61:183-9., 2121. Gordon D, Beastall GH, Thomson JA, Sturrock RD. Androgenic status and sexual function in males with rheumatoid arthritis and ankylosing spondylitis. Q J Med. 1986; 60:671-9.). A more extensive evaluation of this set of patients is necessary to have a clear sense of RA real impact on male fertility.
Most physicians agree that diseases such as RA and AS can cause significant limitations in sexual activity due to diminished desire and impaired mechanical capacity (5252. Gallinaro AL, Akagawa LL, Otuzi MH, Sampaio-Barros PD, Gonçalves CR. Sexual activity in ankylosing spondylitis. Rev Bras Reumatol. 2012; 52:887-91., 5353. Sariyildiz MA, Batmaz I, Dilek B, Inanir A, Bez Y, Tahtasiz M, et al. Relationship of the sexual functions with the clinical parameters, radiological scores and the quality of life in male patients with ankylosing spondylitis. Rheumatol Int. 2013; 33:623-9.). In spite of that, evaluation of sex hormonal levels, seminal analysis and varicocele have shown that AS disease and treatment do not have a major impact in male fertility (77. Paschou S, Voulgari PV, Vrabie IG, Saougou IG, Drosos AA. Fertility and reproduction in male patients with ankylosing spondylitis treated with infliximab. J Rheumatol. 2009; 36:351-4., 2121. Gordon D, Beastall GH, Thomson JA, Sturrock RD. Androgenic status and sexual function in males with rheumatoid arthritis and ankylosing spondylitis. Q J Med. 1986; 60:671-9.
22. Almeida BP, Saad CG, Souza FH, Moraes JC, Nukumizu LA, Viana VS, et al. Testicular Sertoli cell function in ankylosing spondylitis. Clin Rheumatol. 2013; 32:1075-9.-2323. Nukumizu LA, Gonçalves Saad C, Ostensen M, Almeida BP, Cocuzza M, Gonçalves C, et al. Gonadal function in male patients with ankylosing spondylitis. Scand J Rheumatol. 2012; 41:476-81.).
BD is a multisystemic vasculitis with musculoskeletal, muco-cutaneous, ocular, gastrointestinal and neurological findings. The disease is frequently seen in the Mediterranean basin and the Far East. Young adults in their 20s and 30s are typically affected, during their reproductive years, with no gender predilection. However, males usually have a more severe course of the disease and are prone to present eye and other major organ involvement (2828. Uzunaslan D, Saygin C, Hatemi G, Tascilar K, Yazici H. No appreciable decrease in fertility in Behçet’s syndrome. Rheumatology (Oxford). 2014; 53:828-33.). The available literature about BD relationship with infertility is not robust. The disease itself seems to have no impact on fertility potential, but alkylating agents use is associated with its decrement (2626. Fukutani K, Ishida H, Shinohara M, Minowada S, Niijima T, Hijikata K, et al. Suppression of spermatogenesis in patients with Behçet’s disease treated with cyclophosphamide and colchicine. Fertil Steril. 1981; 36:76-80., 2727. Tabbara KF. Chlorambucil in Behçet’s disease. A reappraisal. Ophthalmology. 1983; 90:906-8.). The aggression to testicular tissue by colchicine reported by some authors in BD (2525. Sarica K, Süzer O, Gürler A, Baltaci S, Ozdiler E, Dinçel C. Urological evaluation of Behçet patients and the effect of colchicine on fertility. Eur Urol. 1995; 27:39-42.) was not confirmed in a large gout study (2828. Uzunaslan D, Saygin C, Hatemi G, Tascilar K, Yazici H. No appreciable decrease in fertility in Behçet’s syndrome. Rheumatology (Oxford). 2014; 53:828-33.).
Gout is induced by the deposition of monosodium urate crystals in synovial fluid and other tissues associated with hyperuricemia (5454. Neogi T. Clinical practice. Gout. N Engl J Med. 2011; 364:443-52.). Patient’s with gout are usually older and fertility often is not an issue, as most of them had already constituted their families. Although there is a concern about colchicine impairing reproductive function, decreased fertility has not been found in these patient’s (2929. Yü T. The efficacy of colchicine prophylaxis in articular gout--a reappraisal after 20 years. Semin Arthritis Rheum. 1982; 12:256-64.).
And finally, the modern view of male fertility evaluation gives a new meaning to the term “male infertility management”, which goes beyond the simple identification and elimination of the cause. The andrologist’s therapeutic strategies have changed from a recent past of only attempting to achieve a simple increase in the sperm concentration. We are moving forward and now our main target is to improve the “quality” of spermatozoa and/or preserve fertility (5555. Isidori A, Latini M, Romanelli F. Treatment of male infertility. Contraception. 2005; 72:314-8.). This approach is thus especially recommended for patients with rheumatic diseases, often not considered potentially fertile.
CONCLUSIONS
Rheumatic diseases comprise a heterogeneous Group of diseases with distinct aspects regarding male infertility. SLE clearly affects gonadal function impairing spermatogenesis due to ASA and CYC therapy. Fertility seems to be not affected in BD and AS patients, including patients under anti-TNF therapy. The fertility potential of DM patients may be affected by the disease activity and by alkylating agents. There are also few data regarding RA, however male gonad may be affected by the disease activity and ASA. Gout patients usually do not have any conception desire at the time of disease manifestation and there are no reports of fertility impairment.
A multidisciplinary Group is essential to assess male reproductive health in rheumatic disease patients with a special focus on improving the fertile potential and sexual dysfunction to minimize the disease and treatment damage.
ACKNOWLEDGEMENTS
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP#2014/14806-0 to CAS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ#301411/2009-3 to EB, and#302724/2011-7 to CAS), Federico Foundation (to EB, and CAS) and by Núcleo de Apoio à Pesquisa “Saúde da Criança e do Adolescente” da USP (NAP-CriAd) to CAS.
REFERENCES
-
1Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008; 58:15-25.
-
2Østensen M. New insights into sexual functioning and fertility in rheumatic diseases. Best Pract Res Clin Rheumatol. 2004; 18:219-32.
-
3Clowse ME, Chakravarty E, Costenbader KH, Chambers C, Michaud K. Effects of infertility, pregnancy loss, and patient concerns on family size of women with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2012; 64:668-74.
-
4Gupta R, Deepanjali S, Kumar A, Dadhwal V, Agarwal SK, Pandey RM, et al. A comparative study of pregnancy outcomes and menstrual irregularities in northern Indian patients with systemic lupus erythematosus and rheumatoid arthritis. Rheumatol Int. 2010; 30:1581-5.
-
5Silva CA, Bonfa E, Østensen M. Maintenance of fertility in patients with rheumatic diseases needing antiinflammatory and immunosuppressive drugs.Arthritis Care Res (Hoboken). 2010; 62:1682-90.
-
6Martínez Lopez JA, Loza E, Carmona L. Systematic review on the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy, and breastfeeding). Clin Exp Rheumatol. 2009; 27:678-84.
-
7Paschou S, Voulgari PV, Vrabie IG, Saougou IG, Drosos AA. Fertility and reproduction in male patients with ankylosing spondylitis treated with infliximab. J Rheumatol. 2009; 36:351-4.
-
8Hersh A, von Scheven E, Yelin E. Adult outcomes of childhood-onset rheumatic diseases. Nat Rev Rheumatol. 2011; 7:290-5.
-
9Freire EA, Nepomuceno JD, Maia ID, Ciconelli RM. Doenças reumáticas e infertilidade masculina. Revista Brasileira de Reumatologia. 2006;46:12-20.
-
10Dooley MA, Nair R. Therapy Insight: preserving fertility in cyclophosphamide-treated patients with rheumatic disease. Nat Clin Pract Rheumatol. 2008; 4:250-7.
-
11Miller MH, Urowitz MB, Gladman DD, Killinger DW. Systemic lupus erythematosus in males. Medicine (Baltimore). 1983; 62:327-34.
-
12Soares PM, Borba EF, Bonfa E, Hallak J, Corrêa AL, Silva CA. Gonad evaluation in male systemic lupus erythematosus. Arthritis Rheum. 2007; 56:2352-61.
-
13Suehiro RM, Borba EF, Bonfa E, Okay TS, Cocuzza M, Soares PM, et al. Testicular Sertoli cell function in male systemic lupus erythematosus. Rheumatology (Oxford). 2008; 47:1692-7.
-
14Silva CA, Bonfá E, Borba EF, Braga AP, Soares PM, Moraes AJ, et al. Saúde reprodutiva em homens com lúpus eritematoso sistêmico. Revista Brasileira de Reumatologia. 2009;49:207-22.
-
15Silva CA, Hallak J, Pasqualotto FF, Barba MF, Saito MI, Kiss MH. Gonadal function in male adolescents and young males with juvenile onset systemic lúpus erythematosus. J Rheumatol. 2002; 29:2000-5.
-
16D’Cruz OJ, Haas GG Jr, Reichlin M. Autoantibodies to decondensed sperm nuclear deoxyribonucleic acid in patients with antisperm antibodies and systemic lúpus erythematosus detected by immunofluorescence flow cytometry. Fertil Steril. 1994; 62:834-44.
-
17Shiraishi Y, Shibahara H, Koriyama J, Hirano Y, Okazaki H, Minota S, et al. Incidence of antisperm antibodies in males with systemic autoimmune diseases. Am J Reprod Immunol. 2009; 61:183-9.
-
18Dillon SP, Kurien BT, Li S, Bruner GR, Kaufman KM, Harley JB, et al. Sex chromosome aneuploidies among men with systemic lupus erythematosus. J Autoimmun. 2012; 38:J129-34.
-
19Moraes AJ, Pereira RM, Cocuzza M, Casemiro R, Saito O, Silva CA. Minor sperm abnormalities in young male post-pubertal patients with juvenile dermatomyositis. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al. 2008;41(12):1142-7.
-
20Moraes AJ, Bonfa E, Cocuzza M, Borges CT, Saito O, Silva CA. Gonad evaluation in male dermatomyositis. A pilot study. Clin Exp Rheumatol. 2010; 28:441-2.
-
21Gordon D, Beastall GH, Thomson JA, Sturrock RD. Androgenic status and sexual function in males with rheumatoid arthritis and ankylosing spondylitis. Q J Med. 1986; 60:671-9.
-
22Almeida BP, Saad CG, Souza FH, Moraes JC, Nukumizu LA, Viana VS, et al. Testicular Sertoli cell function in ankylosing spondylitis. Clin Rheumatol. 2013; 32:1075-9.
-
23Nukumizu LA, Gonçalves Saad C, Ostensen M, Almeida BP, Cocuzza M, Gonçalves C, et al. Gonadal function in male patients with ankylosing spondylitis. Scand J Rheumatol. 2012; 41:476-81.
-
24Mizushima Y, Matsumura N, Mori M, Shimizu T, Fukushima B, Mimura Y, et al. Colchicine in Behçet’s disease. Lancet. 1977; 2:1037.
-
25Sarica K, Süzer O, Gürler A, Baltaci S, Ozdiler E, Dinçel C. Urological evaluation of Behçet patients and the effect of colchicine on fertility. Eur Urol. 1995; 27:39-42.
-
26Fukutani K, Ishida H, Shinohara M, Minowada S, Niijima T, Hijikata K, et al. Suppression of spermatogenesis in patients with Behçet’s disease treated with cyclophosphamide and colchicine. Fertil Steril. 1981; 36:76-80.
-
27Tabbara KF. Chlorambucil in Behçet’s disease. A reappraisal. Ophthalmology. 1983; 90:906-8.
-
28Uzunaslan D, Saygin C, Hatemi G, Tascilar K, Yazici H. No appreciable decrease in fertility in Behçet’s syndrome. Rheumatology (Oxford). 2014; 53:828-33.
-
29Yü T. The efficacy of colchicine prophylaxis in articular gout--a reappraisal after 20 years. Semin Arthritis Rheum. 1982; 12:256-64.
-
30Wallace DJ, Hahn B, Dubois EL. Dubois’ lupus erythematosus and related syndromes. 8th ed. Philadelphia, PA: Elsevier/Saunders; 2013. xv, pp. 694.
-
31Vilarinho ST, Costallat LT. Evaluation of the hypothalamic-pituitary-gonadal axis in males with systemic lupus erythematosus. J Rheumatol. 1998; 25:1097-103.
-
32Mok CC, Lau CS. Profile of sex hormones in male patients with systemic lúpus erythematosus. Lupus. 2000; 9:252-7.
-
33Rival C, Guazzone VA, Theas MS, Lustig L. Pathomechanism of autoimune orchitis. Andrologia. 2005; 37:226-7.
-
34Tung KS, Teuscher C. Mechanisms of autoimmune disease in the testis and ovary. Hum Reprod Update. 1995; 1:35-50.
-
35Etteldorf JN, West CD, Pitcock JA, Williams DL. Gonadal function, testicular histology, and meiosis following cyclophosphamide therapy in patients with nephrotic syndrome. J Pediatr. 1976; 88:206-12.
-
36Kumar R, Biggart JD, McEvoy J, McGeown MG. Cyclophosphamide and reproductive function. Lancet. 1972; 1:1212-4.
-
37Qureshi MS, Pennington JH, Goldsmith HJ, Cox PE. Cyclophosphamide therapy and sterility. Lancet. 1972; 2:1290-1.
-
38Romeo C, Ientile R, Santoro G, Impellizzeri P, Turiaco N, Impalà P, et al. Nitric oxide production is increased in the spermatic veins of adolescents with left idiophatic varicocele. J Pediatr Surg. 2001; 36:389-93.
-
39Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005; 95:503-7.
-
40Cocuzza M, Sikka SC, Athayde KS, Agarwal A. Clinical relevance of oxidative stress and sperm chromatin damage in male infertility: an evidence based analysis. Int Braz J Urol. 2007; 33:603-21.
-
41WHO. World Health Organization: WHO Laboratory manual for the examination and processing of human semen - 5th ed. Geneva: WHO Press, 2010.
-
42Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006; 85:629-34.
-
43Arap MA, Vicentini FC, Cocuzza M, Hallak J, Athayde K, Lucon AM, et al. Late hormonal levels, semen parameters, and presence of antisperm antibodies in patients treated for testicular torsion. J Androl. 2007; 28:528-32.
-
44Koide SS, Wang L, Kamada M. Antisperm antibodies associated with infertility: properties and encoding genes of target antigens. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine (New York, NY. 2000;224:123-32.
-
45Haas GG Jr. The inhibitory effect of sperm-associated immunoglobulins on cervical mucus penetration. Fertil Steril. 1986;46:334-7. Erratum in: Fertil Steril 1986; 46:1172.
-
46Marshburn PB, Kutteh WH. The role of antisperm antibodies in infertility. Fertil Steril. 1994; 61:799-811.
-
47Fu L, Xiong DK, Ding XP, Li C, Zhang LY, Ding M, et al. Genetic screening for chromosomal abnormalities and Y chromosome microdeletions in Chinese infertile men. J Assist Reprod Genet. 2012; 29:521-7.
-
48See LC, Kuo CF, Chou IJ, Chiou MJ, Yu KH. Sex- and age-specific incidence of autoimmune rheumatic diseases in the Chinese population: a Taiwan population-based study. Semin Arthritis Rheum. 2013; 43:381-6.
-
49Yu KH, See LC, Kuo CF, Chou IJ, Chou MJ. Prevalence and incidence in patients with autoimmune rheumatic diseases: a nationwide population-based study in Taiwan. Arthritis Care Res (Hoboken). 2013; 65:244-50.
-
50Latta K, von Schnakenburg C, Ehrich JH. A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol. 2001; 16:271-82.
-
51Provost M, Eaton JL, Clowse ME. Fertility and infertility in rheumatoid arthritis. Curr Opin Rheumatol. 2014; 26:308-14.
-
52Gallinaro AL, Akagawa LL, Otuzi MH, Sampaio-Barros PD, Gonçalves CR. Sexual activity in ankylosing spondylitis. Rev Bras Reumatol. 2012; 52:887-91.
-
53Sariyildiz MA, Batmaz I, Dilek B, Inanir A, Bez Y, Tahtasiz M, et al. Relationship of the sexual functions with the clinical parameters, radiological scores and the quality of life in male patients with ankylosing spondylitis. Rheumatol Int. 2013; 33:623-9.
-
54Neogi T. Clinical practice. Gout. N Engl J Med. 2011; 364:443-52.
-
55Isidori A, Latini M, Romanelli F. Treatment of male infertility. Contraception. 2005; 72:314-8.
-
ABREVIATIONS
SLE = Systemic lupus erythematosusAS = Ankylosing spondylitisDM = DermatomyositisRA = Rheumatoid arthritisBD = Behçet diseaseT = TestosteroneFT = Free testosteroneLH = Luteinizing hormoneFSH = Follicle-stimulating hormoneCYC = CyclosphosphamideASA = Antisperm anti-body
Publication Dates
-
Publication in this collection
Jan-Feb 2016
History
-
Received
17 Nov 2014 -
Accepted
17 Sept 2015