Abstracts
A lectin was isolated from the pod saline extract of Caesalpinia tinctoria by dialoconcentration on Centripep-10 and affinity chromatography on chitin column. The purified lectin was partially characterized with respect to its biochemical and structural properties. It contains 8.3 % of carbohydrate and exhibited an agglutinating activity against human erythrocytes (ABO groups). Its amino acid composition was characterized by a great number of acidic and hydrophobic residues and the estimated molecular mass was 12.5 kDa. The presence of only one N-terminal amino acid sequence (D¹-V-P-A-Y-V-Y-V-H-F10-G-F-G-E-E-H-R -D-V-F20-D), showed the homogeneity of the purified lectin. The far-ultraviolet circular dichroism (CD) spectrum of lectin indicated that it contains 10 % a-helix, 38 % b-sheet, 28 % unordered form and 6 % of P II (poly-L-proline II helix conformation).
circular dichroism; Leguminosae; plant defense; pod; secondary structure
Isolou-se uma lectina a partir do extrato salino da fava dos frutos de Caesalpinia tinctoria mediante dialofiltração e cromatografia de afinidade em coluna de quitina. A lectina purificada foi caracterizada parcialmente com relação às suas propriedades bioquímicas e estruturais, e classificada como um glicopeptídio contendo 8,3 % de carboidratos, aglutinando hemácias do grupo ABO. Sua composição em aminoácidos foi caracterizada por grande número de resíduos ácidos e hidrofóbicos, e sua massa molecular, estimada em 12,5 kDa. A análise do grupo N-terminal mostrou a homogeneidade da lectina, cuja seqüência dos 21 resíduos iniciais é a seguinte: D¹-V-P-A-Y-V-Y-V-H-F10-G-F-G-E-E-H-R-D-V-F 20-D. O espectro de dicroísmo circular da lectina na região do UV distante indicou a presença de 10 % de a-hélice; 38 % de folhas b; 19 % de voltas b; 28 % de estruturas desordenadas e 6 % da conformação P II (hélice poli-L-prolina II).
defesa de plantas; dicroísmo circular; estrutura secundária; fava; Leguminosae
SHORT COMMUNICATION
Purification and partial characterization of a lectin from Caesalpinia tinctoria Domb, ex Dc fruits
Purificação e caracterização parcial de uma lectina extraída do fruto de Caesalpinia tinctoria Domb, ex Dc
Marli Lourdes de OliveiraI; Leila Maria BeltraminiII; Salvatore Giovanni de SimoneIII; Maria Helena Nasser BrumanoI; Rosemeire Aparecida Silva-LuccaIV; Marcelo Kiyoshi Kian NakaemaV; Christiano Vieira PiresI; Maria Goreti de Almeida OliveiraI, * * Corresponding author: malmeida@mail.ufv.br
IDepartamento de Bioquímica e Biologia Molecular/Universidade Federal de Viçosa, 36571-000, Viçosa, MG, Brasil
IIDepartamento de Física e Informática, Instituto de Física de São Carlos-SP
IIILaboratório de Química de Proteínas/FIOCRUZ, Rio de Janeiro-RJ
IVCentro de Engenharias e Ciências Exatas da UNIOESTE, Toledo-PR
VInstituto de Física da UNICAMP, Campinas-SP
ABSTRACT
A lectin was isolated from the pod saline extract of Caesalpinia tinctoria by dialoconcentration on Centripep-10 and affinity chromatography on chitin column. The purified lectin was partially characterized with respect to its biochemical and structural properties. It contains 8.3 % of carbohydrate and exhibited an agglutinating activity against human erythrocytes (ABO groups). Its amino acid composition was characterized by a great number of acidic and hydrophobic residues and the estimated molecular mass was 12.5 kDa. The presence of only one N-terminal amino acid sequence (D1-V-P-A-Y-V-Y-V-H-F10-G-F-G-E-E-H-R -D-V-F20-D), showed the homogeneity of the purified lectin. The far-ultraviolet circular dichroism (CD) spectrum of lectin indicated that it contains 10 % a-helix, 38 % b-sheet, 28 % unordered form and 6 % of PII (poly-L-proline II helix conformation).
Key words: circular dichroism, Leguminosae, plant defense, pod, secondary structure.
RESUMO
Isolou-se uma lectina a partir do extrato salino da fava dos frutos de Caesalpinia tinctoria mediante dialofiltração e cromatografia de afinidade em coluna de quitina. A lectina purificada foi caracterizada parcialmente com relação às suas propriedades bioquímicas e estruturais, e classificada como um glicopeptídio contendo 8,3 % de carboidratos, aglutinando hemácias do grupo ABO. Sua composição em aminoácidos foi caracterizada por grande número de resíduos ácidos e hidrofóbicos, e sua massa molecular, estimada em 12,5 kDa. A análise do grupo N-terminal mostrou a homogeneidade da lectina, cuja seqüência dos 21 resíduos iniciais é a seguinte: D1-V-P-A-Y-V-Y-V-H-F10-G-F-G-E-E-H-R-D-V-F 20-D. O espectro de dicroísmo circular da lectina na região do UV distante indicou a presença de 10 % de a-hélice; 38 % de folhas b; 19 % de voltas b; 28 % de estruturas desordenadas e 6 % da conformação PII (hélice poli-L-prolina II).
Palavras-chave: defesa de plantas, dicroísmo circular, estrutura secundária, fava, Leguminosae.
Plant lectins are a heterogeneous group of proteins or glycoproteins that share in common their ability to bind specific sugar residues and to agglutinate cells. Because lectins are found in many different species and in many different organs and tissues of plants, it is assumed that they play fundamental biological roles, including part of their defense mechanisms (Peumans and Van Damme, 1995). Seeds, particularly those of the Leguminosae, are rich sources of lectins (Carlini and Grossi-de-Sá, 2002). In this communication, we describe the characterization of a novel non-seed legume lectin isolated from pod saline extract of Caesalpinia tinctoria fruits.
Fruits from Caesalpinia tinctoria were collected near São Carlos (São Paulo, Brazil). Dried seeds and pods were ground separately and suspended in 150 mmol.L-1 phosphate-buffered saline (PBS), pH 7.4, (1:10, w/v), containing 1.5 % polyvinylpolypyrrolidone. The resulting suspensions were stirred for 12 h at 4oC, centrifuged at 17,600 gn for 30 min at 4oC, and the supernatant solutions (crude extract) were used for determining the protein content, haemagglutinating activity and inhibition for the following sugars: D-glucose, D-mannose, a-methyl-manoside, D-galactose, D-xilose, L-ramnose, L-fucose, L-arabinose, sucrose, a-lactose, maltose, N-acetylglucosamine, D-galactosamine, N-acetyl-D-galactosamine. Protein concentration was determined according to Bradford (1976), using bovine serum albumin as standard. Agglutination assays were carried out in microtiters plates containing 40 µL of a 2 % suspension of erythrocytes and 40 µL of crude extracts or lectin solutions (series of 1:2 dilutions). Agglutination, visible to the naked eye, was monitored after plates had been left for 30 min at 22oC. Since agglutination activity was found only in pod extracts, we performed a purification of this material. Crude extract containing 550 mg.mL-1 of protein was ten times diluted in PBS and a volume of 2 mL from this solution was dialoconcentrated through Centripep-10 concentrators from Amicon. The microconcentrators that contained the recovered material were filled to 2 mL of PBS, and after two new concentrations under these conditions, a volume of 4 mL of the recovered fraction was applied to a chitin column (11.5 x 2.5 cm) equilibrated with 10 mmol.L-1 PBS, pH 7.4. Lectin was eluted from the affinity resin with 0.5 mol.L-1 NH4OH and collected in 5 mL fractions on tubes containing sodium acetate buffer, pH 5.5. This effluent showing haemagglutinating activity was concentrated to 6 mL by ultrafiltration under pressure (Amicon YM 1,000 membrane), stored at -18oC, and used for analysis. The amino acid composition was determined as described by Cohen and Strydom (1988). Amino-terminal sequence analysis was carried out on a gas-phase protein sequence system (Model PSQ-1, Shimadzu), according to De Simone et al. (1994). Total soluble sugars were quantified by the phenol sulphuric acid method (Dubois et al., 1956) using glucose as a standard. CD spectra were recorded using a Jasco J-720 spectropolarimeter over a wavelength range of 202-380 nm. Analysis of the far-ultraviolet CD spectrum in terms of the secondary structure content was performed using the Self-consistent method (SELCON), developed by Sreerama and Woody (1994).
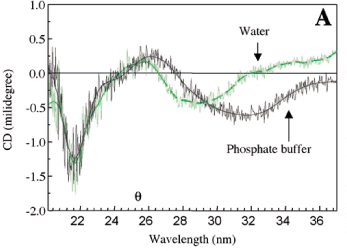
Absence of haemagglutinating activity in the saline extract of Caesalpinia tinctoria seeds agree with results reported by several Leguminosae seeds (Toms and Western, 1971). The pod saline extract agglutinates erythrocytes even at 1:128 dilution, and also the agglutination was not inhibited by the sugars tested. When whole fruits of Caesalpinia tinctoria were imbibed in water or PBS, agglutinating activity was detected in the imbibition solution after 30 min, indicating that haemagglutinating substances were released to the medium. The haemagglutinating activity observed in the pod extract still remained after its exposure to 90oC for 15 min, whereas purified lectin lost its agglutinating activity at 80oC for 5 min, indicating that this isolated protein was not responsible for the total haemagglutinating activity in the crude extract. The lectin was retained in a chitin resin (figure 1) which is a polysaccharide consisted of N-acetylglucosamine residues. This suggests that its haemagglutinating activity can be inhibited by N-acetylglucosamine oligomers as reported for Chelidonium majus lectin (Peumans et al., 1985). As shown in table 1, about 112 mg of pure lectin could be recovered from 780 mg of the saline extract with a 14 % yield. The minimum amount of protein required for agglutination of human erythrocytes reduced by 26 times compared with that of the crude extract. The amino acid composition of lectin (table 2) was characterized by a high content of acidic and hydrophobic amino acids. Cyst(e)ine and methionine were almost absent, similar to what has been observed in other leguminous sources (Goldstein and Poretz, 1986). The molecular mass estimated from its amino acid composition was 12.5 kDa. It was found only one N-terminal amino acid sequence (D1-V-P-A-Y-V-Y-V-H-F10-G-F-G-E-E-H-R-D-V -F20-D) for purified lectin. Sugar determination indicated a 8.3 % of carbohydrates, suggesting that the lectin is a glycoprotein. The CD spectra of lectin in phosphate buffer at pH 7.0 and water are presented in figure 2A. They were very similar in the far-UV region (200-250 nm) and characterized by a minimum at 218 nm. In the near-UV region (260-360 nm) the CD spectra had a minimum at 290 nm in water and at 332 nm in phosphate buffer, indicating spectral changes as a function of pH. These changes can be explained by the presence of aromatic amino acids, prosthetic groups such as phenols, or iron and cupper ions (Cantor and Schimmel, 1980). The far-UV CD spectrum of lectin is shown in figure 2B. The estimated secondary structure content was 10 % a-helix, 38 % b-sheet, 19 % b-turns, 28 % unordered form and 6 % of PII. High amounts of b-sheet strucuture are found in a wide variety of the legume lectins (Loris et al., 1998). Based on the N-terminal sequence criteria, the purity of the isolated lectin was demonstrated, however it does present a ligant that can be phenols or tannins, which are abundant on these pods (Galvez et al., 1997). This conclusion is based on a positive qualitative test for total phenols and also due to the pH-induced changes in the near-UV CD region, that may result from the ionization of these groups. The presence of lectin in the Caesalpinia tinctoria pods and the diffusion of haemagglutinating substances from the fruits upon its imbibition suggest a possibly protection role against pathogens and insects for the glycoprotein isolated from this indehiscent fruit.
Acknowledgements: We thank to CAPES/PICD program for the doctoral fellowship to M.L.O. and to CNPq, FAPESP and FINEP for financial support.
Received: 05/04/2003
Accepted: 02/06/2003
- Alonzo N, Hirs CHW (1968) Automation of sample application in amino acid analyzers. Anal. Biochem. 23:272-288.
- Bradford MM(1976) A rapid and sensitive method for the quantitation of microgram protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254.
- Cantor CR, Schimmel PR (1980) Part II: Techniques for the study of biological structure and function: Spectroscopic Analysis of Biopolymers. Biophysical Chemistry, pp. 846-910. WH Freeman and Company, New York.
- Carlini CR, Grossi-de-Sá MF (2002) Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides. Toxicon 40:1515-1539.
- Cohen SA, Strydom DJ (1988) Amino acid analysis utilizing phenylisothiocyanate derivatives. Anal. Biochem. 174:1-16.
- De Simone SG, Santos R, Araujo MF, Pinho RT (1994) Preparative isolation of the lectin jacalin by anion-exchange high-performance liquid chromatography. J. Chromatogr. 688:357-362.
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356.
- Galvez JMG, Rieddl B, Conner AH (1997) Analytical studies on tara tannins. Holzforschung 51:235-243.
- Goldstein IJ, Poretz RD (1986) Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins. In: Liener, IE, Sharon, N, Goldstein, IJ (eds), The Lectins: properties, functions, and applications in biology and medicine, pp.33-247. Academic Press, Orlando.
- Loris R, Hamelryck T, Bouckaert J, Wyns L (1998) Legume lectin structure. Biochim. Biophys. Acta 1383:9-36.
- Peumans WJ, de Ley M, Stinissen HM, Broekaert WF (1985) Isolation and partial characteriztion of a new lectin from seeds of the greater celandine (Chelidonium majus). Plant Physiol. 78:379-383.
- Peumans WJ, Van Damme EJM (1995) Lectin as plant defense proteins. Plant Physiol. 109:347-352.
- Sreerama N, Woody RW (1994) Poly(Pro)II helix in globular proteins: Identification and circular dichroic analysis. Biochemistry 33:10022-10025.
- Toms GC, Western A (1971) Phytohaemagglutinins. In: Harbone, JB, Boulter, D, Turner, BL (eds), Chemotaxonomy of the Leguminosae, pp.367-462. Academic Press Inc, London.
Publication Dates
-
Publication in this collection
26 Sept 2003 -
Date of issue
Aug 2003
History
-
Accepted
02 June 2003 -
Received
05 Apr 2003