ABSTRACT
PURPOSE:
To assess and compare the histopathological effects of ozone therapy and/or methylprednisolone (MPS) treatment on regeneration after crush type sciatic nerve injury.
METHODS:
Forty Sprague-Dawley male rats were randomly allocated into four groups. Four groups received the following regimens intraperitoneally every day for 14 days after formation of crush type injury on sciatic nerve: Group I: ozone (20mcg/ml); Group II: methylprednisolone (2mg/kg); Group III: ozone (20 mcg/ml) and methylprednisolone (2mg/kg); Group IV: isotonic saline (0.9%). The histomorphological evaluation was made after biopsies were obtained from the sites of injury.
RESULTS:
Significant differences were noted between groups in terms of degeneration (p=0.019), nerve sheath cell atrophy (p=0.012), intraneural inflammatory cellular infiltration (p=0.002), perineural granulation tissue formation (p=0.019), perineural vascular proliferation (p=0.004), perineural inflammatory cellular infiltration (p<0.001) and inflammation in peripheral tissue (p=0.006). Degeneration was remarkably low in Group III, while no change in nerve sheath cell was noted in Group II.
CONCLUSION:
The combined use of methylprednisolone and ozone treatment can have beneficial effects for regeneration after crush type nerve injury.
Key words:
Ozone; Methylprednisolone; Nerve Regenertaions; Rats
Introduction
Crush type peripheral nerve injury is a frequent type of nerve injury and insufficient treatment is associated with sensory, motor or autonomic functional deficits. Regeneration and repair process follow nerve injury can be impaired due to the oxidative stress11 Ozbay I, Ital I, Kucur C, Akcilar R, Deger A, Aktas S, Oghan F: Effects of ozone therapy on facial nerve regeneration. Braz J Otorhinolaryngol. 2016 Apr 22. doi: 10.1016/j.bjorl.2016.02.009. [Epub ahead of print]
https://doi.org/10.1016/j.bjorl.2016.02....
,22 Yüce S, Cemal Gökçe E, Iskdemir A, Koç ER, Cemil DB, Gökçe A, Sargon MF. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann Plast Surg. 2015;74:684-92. doi: 10.1097/SAP.0000000000000026.
https://doi.org/10.1097/SAP.000000000000...
. Antioxidants may alleviate the adverse effects of free oxygen radicals on nerve regeneration33 Lenaz G, Genova ML. Mobility and function of coenzyme Q(ubiquinone) in the mitochondrial respiratory chain. Biochim Biophys Acta. 2009;1787:563-73. doi: 10.1016/j.bbabio.2009.02.019.
https://doi.org/10.1016/j.bbabio.2009.02...
,44 Thomas DA, Ren K, Besse D, Ruda MA, Dubner R. Application of nitric oxide synthase inhibitor, N omega-nitro-l-arginine methylester, on injured nerve attenuates neuropathy-induced thermalhyperalgesia in rats. Neurosci Lett. 1996;210:124-6. PMID: 8783289..
Ozone is a powerful oxidant with antibacterial, antifungal and antiviral effects55 Al-Dalain SM, Martinez G, Candelario-Jalil E, Menendez S, Re L, Giuliani A, Leon OS. Ozone treatment reduces markers of oxidative and endothelial damage in an experimental diabetes model in rats. Pharmacol Res. 2001;44:391-6. doi: 10.1006/phrs.2001.0867.
https://doi.org/10.1006/phrs.2001.0867...
. It enhances the oxygenation of tissues and triggers the secretion of cytokines66 Batinjan G, Filipovic Zore I, Vuletic M, Rupic I. The use of ozone in the prevention of osteoradionecrosis of the jaw. Saudi Med J. 2014;35:1260-3. PMID: 25316473.. It has been used as a therapeutic agent for chronic and diabetic wounds77 Martinez-Sanchez G, Al-Dalain SM, Menendez S, Re L, Giuliani A, Candelario-Jalil E, Alvarez H, Fernández-Montequín JI, León OS. Therapeutic efficacy of ozone in patients with diabetic foot. Eur J Pharmacol. 2005;523:151-61. doi: 10.1016/j.ejphar.2005.08.020.
https://doi.org/10.1016/j.ejphar.2005.08...
. Ozone exerts its beneficial effects by supression of infection, regulation of inflammation and increasing local oxygen tension at the site of injury88 Kim HS, Noh SU, Han YW, Kim KM, Kang H, Kim HO, Park YM. Therapeutic effects of topical application of ozone on acute cutaneous wound healing. J Korean Med Sci. 2009;24:368-74. doi: 10.3346/jkms.2009.24.3.368.
https://doi.org/10.3346/jkms.2009.24.3.3...
,99 Lim Y, Phung AD, Corbacho AM, Aung HH, Maioli E, Reznick AZ, Cross CE, Davis PA, Valacchi G. Modulation of cutaneous wound healing by ozone: differences between young and aged mice. Toxicol Lett. 2006;160:127-34. doi: 10.1016/j.toxlet.2005.06.013.
https://doi.org/10.1016/j.toxlet.2005.06...
. Ozone is particularly useful for infected and necrotic wounds and poorly oxygenated tissues66 Batinjan G, Filipovic Zore I, Vuletic M, Rupic I. The use of ozone in the prevention of osteoradionecrosis of the jaw. Saudi Med J. 2014;35:1260-3. PMID: 25316473..
Methylprednisolone (MPS) is an effective neuroprotective agent tested in controlled, multicentric, clinical trials. It inhibits lipid peroxidation and reduces the posttraumatic degenerative changes after neural injury1010 Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A. Coenzyme Q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278:28220-8. doi: 10.1074/jbc.M302297200.
https://doi.org/10.1074/jbc.M302297200...
. However, the use of corticosteroids has been debateful in recent years1111 Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335-43. doi: 10.1016/j.spinee.2005.11.001.
https://doi.org/10.1016/j.spinee.2005.11...
,1212 Al-Bishri A, Dahlin L, Sunzel B, Rosenquist J. Systemic betamethasone accelerates functional recovery after acrush injury to rat sciatic nerve. J Oral Maxillofac Surg. 2005;63:973-7. PMID: 16003625..
A rat sciatic nerve model is frequently used in the evaluation of functional healing after peripheral nerve injuries. After nerve injury, ischemic and inflammatory processes begin and treatments can be used to decrease the effects of these processes. Efforts have been spent to analyze the impacts of various agents on healing22 Yüce S, Cemal Gökçe E, Iskdemir A, Koç ER, Cemil DB, Gökçe A, Sargon MF. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann Plast Surg. 2015;74:684-92. doi: 10.1097/SAP.0000000000000026.
https://doi.org/10.1097/SAP.000000000000...
.
Our aim was to evaluate and compare the histopathological effects of ozone therapy and MPS treatment on regeneration after crush type sciatic nerve injury.
Methods
This experimental study was carried out with the guidelines of local Experimental Animal Laboratory after the approval of the local Animal Care and Use Committee (19.11.2015-2015/114).
Forty Sprague-Dawley male rats (weighing 300-350 g) were randomly allocated into four groups (n=10 for each group). Animals were maintained from the same center and housed in individual cages with free access to water and animal chow. The animals were maintained in a constant 12 h light/12 h dark cycle at constant room temperature of 21°C and humidity of 60%.
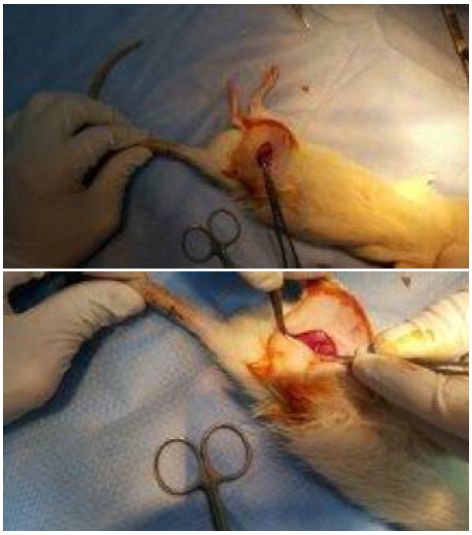
After induction of anesthesia by ketamine hydrochloride (90 mg/kg) (Ketalar(r), Pfizer Pharmaceuticals Ltd, Istanbul, Turkey) and Xylazine hydrochloride (10 mg/kg) (Alfazyne 2%, Alfasan International B.V., Woerden, Netherlands) intraperitoneally, animals were placed in prone position. The operative field was shaved and sterilely prepared. An oblique incision was made 2-3 mm distal to the level right hip joint. The dissection proceeded subcutaneously until biceps femoris muscle. Blunt dissection was performed along the posterior margin of femur until identification of the sciatic nerve. Under microscopic view, crush type injury was formed by compression of the right sciatic nerve with jeweler's forceps for 30 seconds. The site of injury was marked by 5/0 silk stitches in the muscular tissue around the dissection area with. None of the rats died during intervention for experimental injury. Primary repair of biceps muscle was made by means of 5/0 round vicryl sutures (Surgisorb, Sutures Limited, Wrexham, Wales, UK). Skin was sutured continuously with 4/0 sharp silk (Neosilk, Setpa Medical Equipment Ltd., Izmir, Turkey). Surgical procedure was finished after closure and local dressing of the wound (Figure 1).
Four groups received the following regimens after formation of crush type injury: Group I: Ozone (20 mcg/ml) was applied intraperitoneally every day for 14 days; Group II: Methylprednisolone (2 mg/kg) was administered daily through intraperitoneal route; Group III: Ozone (20 mcg/ml) and methylprednisolone (2 mg/kg) were administered daily for 14 days using different syringes; Group IV: Isotonic saline (0.9%) was administered intraperitoneally for 14 days.
On the 14th day, biopsies were obtained from all rats under general anesthesia induced by ketamine (80 mg/kg). A tissue sample was obtained from the sciatic nerve with the guidance of the site previously marked with sutures during formation of crush injury. Specimen were maintained in 10% formaldehyde until histopathological examination was performed. After all the surgical procedures were finished, eutanasia was accomplished by decapitation.
Histopathological examination
Samples maintained in 10 % formaldehyde are stained with Harris hematoxylin stain. Microscopic examinations were performed under x 40, x 100, x 200 and x 400 magnifications using Zeiss microscope (Carl Zeiss Microscopy, Jena, Germany), respectively. Images were captured from fields under these magnifications.
Outcome parameters
Histological variables under investigation consisted of degeneration; intraneural cellular inflammation, fibrosis, granulation tissue formation, vascular proliferation, cellular inflammation in perineurium; muscular injury and injury in the peripheral tissues.
Statistical analysis
Analysis of data was made with IBM Statistical Package for Social Sciences (SPSS) software version 20.0 for Windows (SPSS Inc., Chicago, IL, USA). Comparison of categorical variables was carried out with Pearson Chi-Square test. Confidence interval was 95% and p value less than 0.05 was considered as statistically significant.
Results
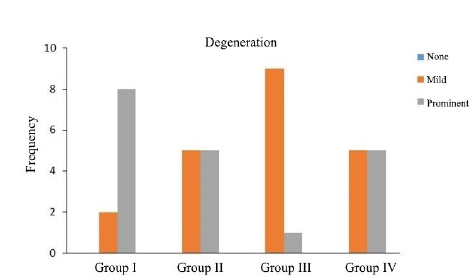
As shown in Table 1, there were significant differences between groups in terms of degeneration (p=0.019), nerve sheath cell atrophy (p=0.012), intraneural inflammatory cellular infiltration (p=0.002), perineural granulation tissue formation (p=0.019), perineural vascular proliferation (p=0.004), perineural inflammatory cellular infiltration (p<0.001) and inflammation in peripheral tissue (p=0.006). No remarkable differences were detected between groups with respect to perineural fibrosis and muscular injury.
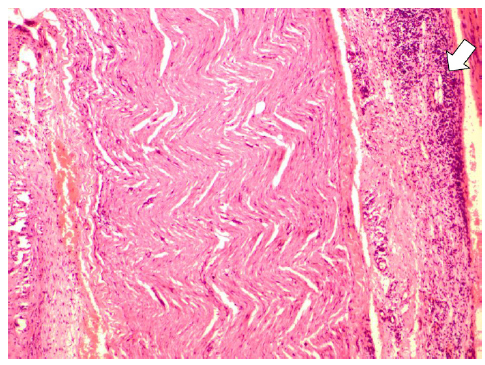
Perineural inflammatory cells were more frequently observed in Group II (Figure 2). Degeneration was remarkably low in Group III, while no change in nerve sheath cell was noted in Group II (Figure 3). Intraneural inflammatory cell infiltration was highest in Group I and lowest in Group II (Figure 4). Perineural granulation tissue formation was most evident in Group III and least obvious in Group II (Figure 5). Perineural vascular proliferation and inflammation in peripheral tissue were most noteworthy in Group III.
Discussion
The present study was performed to evaluate comparatively the histomorphological changes that occur after ozone therapy and/or MPS treatment following crush injury of the sciatic nerve in an experimental rat model. Our results yielded that degeneration was lowest while perineural vascular proliferation and granulation tissue formation as well as peripheral inflammation was remarkable after treatment with ozone and MPS. These findings imply that combined treatment with MPS and ozone may yield beneficial effects after crush injury of the nerve.
In contrast to the central nervous system, peripheral nerves are able to regenerate injury1313 Noorafshan A, Omidi A, Karbalay-Doust S. Curcumin protects the dorsal root ganglion and sciatic nerve after crush in rat. Pathol Res Pract. 2011;207:577-82. doi: 10.1016/j.prp.2011.06.011.
https://doi.org/10.1016/j.prp.2011.06.01...
. Axonotmesis can be seen after crush injury of the nerves and in spite of the integrity of endoneurial sheath, axonal destruction as well as Wallerian degeneration can be noted distal to the injury. Even though functional outcome can be satisfactory attributed to the spontaneous regeneration in the distal nerve stump, failure may be observed due to nerve cell death and end organ atrophy. Furthermore, scar tissue existing on the injury can mechanically block axonal extension and may adversely influence the process of recovery1414 Luis AL, Amado S, Geuna S, Rodrigues JM, Simões MJ, Santos JD, Fregnan F, Raimondo S, Veloso AP, Ferreira AJ, Armada-da-Silva PA, Varejão AS, Maurício AC. Long-term functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. J Neurosci Methods. 2007;163:92-104. doi: 10.1016/j.jneumeth.2007.02.017.
https://doi.org/10.1016/j.jneumeth.2007....
.
Crush injury of the peripheral nerves is followed by interruption of mechanical transmission and microvasculation of the nerve. Reperfusion results in accumulation of oxygen and nutrients, which subsequently enhances the formation of free radicals. Free radicals lead to lipid peroxidation which has deleterious effects on tissue22 Yüce S, Cemal Gökçe E, Iskdemir A, Koç ER, Cemil DB, Gökçe A, Sargon MF. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann Plast Surg. 2015;74:684-92. doi: 10.1097/SAP.0000000000000026.
https://doi.org/10.1097/SAP.000000000000...
,1515 Yilmaz Z, Senoglu M, Kurutas EB, Ciralik H, Ozbag D. Neuroprotective effects of mannitol and vitamin C on crush injury of sciatic nerve; an experimental rat study. J Neurol Sci (Turkish). 2011;28:538-51.. The cumulative effect of ischemic and mechanical processes is supposed to be more prominent than their own influences22 Yüce S, Cemal Gökçe E, Iskdemir A, Koç ER, Cemil DB, Gökçe A, Sargon MF. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann Plast Surg. 2015;74:684-92. doi: 10.1097/SAP.0000000000000026.
https://doi.org/10.1097/SAP.000000000000...
,1515 Yilmaz Z, Senoglu M, Kurutas EB, Ciralik H, Ozbag D. Neuroprotective effects of mannitol and vitamin C on crush injury of sciatic nerve; an experimental rat study. J Neurol Sci (Turkish). 2011;28:538-51.. Treatment with antioxidant and anti-inflammatory agents yielded promising results for regeneration of peripheral nerves1515 Yilmaz Z, Senoglu M, Kurutas EB, Ciralik H, Ozbag D. Neuroprotective effects of mannitol and vitamin C on crush injury of sciatic nerve; an experimental rat study. J Neurol Sci (Turkish). 2011;28:538-51.. The basis for exogeneous administration of various biological substances to the microenvironment around the damaged axon is to facilitate the regeneration of nerve at cellular level22 Yüce S, Cemal Gökçe E, Iskdemir A, Koç ER, Cemil DB, Gökçe A, Sargon MF. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann Plast Surg. 2015;74:684-92. doi: 10.1097/SAP.0000000000000026.
https://doi.org/10.1097/SAP.000000000000...
. Assessment of the effectiveness of therapeutic agents administered subsequent to the neural injury is critical to discriminate various strategies for follow-up of recovery following neural injury1616 Muir GD, Webb AA. Mini-review: assessment of behavioural recovery following spinal cord injury in rats. Eur J Neurosci. 2000;12:3079-86. PMID: 10998091..
The mechanisms of neuroprotective effect of MPS are obscure. Previously, use of corticosteroids was based on their capability of reduction of edema after spinal cord edema. Recently, additional mechanisms of MPSs such as inhibition of lipid peroxidation and inflammatory cytokines, preservation of calcium homeostasis, restoration of local blood flow and modulation of inflammation have been suggested1717 Pereira JE, Costa LM, Cabrita AM, Couto PA, Filipe VM, Magalhães LG, Fornaro M, Di Scipio F, Geuna S, Maurício AC, Varejão AS. Methylprednisolone fails to improve functional and histological outcome following spinal cord injury in rats. Exp Neurol. 2009;220:71-81. doi: 10.1016/j.expneurol.2009.07.030.
https://doi.org/10.1016/j.expneurol.2009...
. Recently, MPSs were found to alleviate the apoptosis of oligodendrocytes after injury without affecting neuronal survival1818 Lee JM, Yan P, Xiao Q, Chen S, Lee KY, Hsu CY, Xu J. Methylprednisolone protects oligodendrocytes but not neurons after spinalcordinjury. J Neurosci. 2008;28:3141-9. doi: 10.1523/JNEUROSCI.5547-07.2008.
https://doi.org/10.1523/JNEUROSCI.5547-0...
.
Pereira et al. suggested that MPS treatment did not have a favorable effect on neural regeneration after spinal cord injury1717 Pereira JE, Costa LM, Cabrita AM, Couto PA, Filipe VM, Magalhães LG, Fornaro M, Di Scipio F, Geuna S, Maurício AC, Varejão AS. Methylprednisolone fails to improve functional and histological outcome following spinal cord injury in rats. Exp Neurol. 2009;220:71-81. doi: 10.1016/j.expneurol.2009.07.030.
https://doi.org/10.1016/j.expneurol.2009...
. A recent publication has shown that MPS treatment was associated with mild neuroprotection without improvement of functional recovery1919 Mu X, Azbill RD, Springer JE. Riluzole and methylprednisolone combined treatment improves functional recovery in traumatic spinal cord injury. J. Neurotrauma. 2000;17:773-80. doi: 10.1089/neu.2000.17.773.
https://doi.org/10.1089/neu.2000.17.773...
. However, we noted that histomorphological changes after crush injury were less prominent in nerve sheath cells, and intraneural inflammation as well as perineural granulation tissue formation was reduced in rats receiving MPS. Therefore, our results remind that MPS treatment may have remarkable effects on regeneration process after peripheral nerve injury.
Ozone can be administered through many routes depending on the site of lesion and clinical picture2020 Bocci V. Biological and clinical effects of ozone. Has ozone therapy a future in medicine? Br J Biomed Sci. 1999;56:270-9. PMID: 10795372.,2121 Valacchi G, Fortino V, Bocci V. The dual action of ozone on the skin. Br J Dermatol. 2005;153:1096-1100. doi: 10.1111/j.1365-2133.2005.06939.x.
https://doi.org/10.1111/j.1365-2133.2005...
. Ozone may promote angiogenesis and re-establishment of vascularization in the connective tissue following injury. Thus, oxygen and nutrient supply will occur more effectively at the wound site, facilitating the healing of the wound88 Kim HS, Noh SU, Han YW, Kim KM, Kang H, Kim HO, Park YM. Therapeutic effects of topical application of ozone on acute cutaneous wound healing. J Korean Med Sci. 2009;24:368-74. doi: 10.3346/jkms.2009.24.3.368.
https://doi.org/10.3346/jkms.2009.24.3.3...
. Antimicrobial feature of ozone in conjunction with its immunomodulatory action can be responsible for these effects99 Lim Y, Phung AD, Corbacho AM, Aung HH, Maioli E, Reznick AZ, Cross CE, Davis PA, Valacchi G. Modulation of cutaneous wound healing by ozone: differences between young and aged mice. Toxicol Lett. 2006;160:127-34. doi: 10.1016/j.toxlet.2005.06.013.
https://doi.org/10.1016/j.toxlet.2005.06...
. It can be postulated that ozone may constitute a safe and effective therapeutic alternative attributed to its impacts on oxygen metabolism, cellular energy and antioxidant defense mechanisms as well as vascular system11 Ozbay I, Ital I, Kucur C, Akcilar R, Deger A, Aktas S, Oghan F: Effects of ozone therapy on facial nerve regeneration. Braz J Otorhinolaryngol. 2016 Apr 22. doi: 10.1016/j.bjorl.2016.02.009. [Epub ahead of print]
https://doi.org/10.1016/j.bjorl.2016.02....
.
Analysis of our data has shown that degeneration and intraneural inflammatory cell infiltration were more obvious in rats receiving ozone therapy only after crush injury. Even though Ozbay et al.11 Ozbay I, Ital I, Kucur C, Akcilar R, Deger A, Aktas S, Oghan F: Effects of ozone therapy on facial nerve regeneration. Braz J Otorhinolaryngol. 2016 Apr 22. doi: 10.1016/j.bjorl.2016.02.009. [Epub ahead of print]
https://doi.org/10.1016/j.bjorl.2016.02....
suggested that ozone treatment can be a promising option for improvement of healing after crush type nerve injury, we suggest that further histomorphological studies are warranted to understand the mechanisms of action of ozone therapy and MPS treatment. Results of the present study indicated that MPS and ozone can have synergistic effects for enhancement of regeneration and healing after injury of peripheral nerves.
Healing and regeneration after injury is a multi-dimensional and complex process that occurs with interaction of many factors. Therefore, outcomes of the present study are vulnerable to be influenced by many variables. We observed various histomorphological effects of ozone and MPS on inflammation and degeneration. Additive and positive effects of ozone and MPS may be useful to establish new strategies for improvement of outcomes after nerve injury. Histomorphological studies are particularly remarkable since they allow insight for regeneration mechanisms.
On the other hand, it must be remembered that adjunctive therapeutic options are of choice if comprehensive management strategies fail. Further scientific evidence is essential to establish guidelines and strategies for treatment of crush type nerve injury. Disadvantages and contraindications for every treatment option must be taken into account during determination of regimen.
Main weaknesses of the current trial consist of experimental design, lack of a sham group and possible impacts of environmental and technical factors which may influence the outcomes under investigation. Main strengths of our study are comparative histomorphological evaluation and adequate sample size.
Conclusions
Combined use of methylprednisolone and ozone treatment can have beneficial effects for regeneration after crush type nerve injury. These favorable effects were supported by evidence from histopathological evaluation.
References
-
1Ozbay I, Ital I, Kucur C, Akcilar R, Deger A, Aktas S, Oghan F: Effects of ozone therapy on facial nerve regeneration. Braz J Otorhinolaryngol. 2016 Apr 22. doi: 10.1016/j.bjorl.2016.02.009. [Epub ahead of print]
» https://doi.org/10.1016/j.bjorl.2016.02.009 -
2Yüce S, Cemal Gökçe E, Iskdemir A, Koç ER, Cemil DB, Gökçe A, Sargon MF. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann Plast Surg. 2015;74:684-92. doi: 10.1097/SAP.0000000000000026.
» https://doi.org/10.1097/SAP.0000000000000026 -
3Lenaz G, Genova ML. Mobility and function of coenzyme Q(ubiquinone) in the mitochondrial respiratory chain. Biochim Biophys Acta. 2009;1787:563-73. doi: 10.1016/j.bbabio.2009.02.019.
» https://doi.org/10.1016/j.bbabio.2009.02.019 -
4Thomas DA, Ren K, Besse D, Ruda MA, Dubner R. Application of nitric oxide synthase inhibitor, N omega-nitro-l-arginine methylester, on injured nerve attenuates neuropathy-induced thermalhyperalgesia in rats. Neurosci Lett. 1996;210:124-6. PMID: 8783289.
-
5Al-Dalain SM, Martinez G, Candelario-Jalil E, Menendez S, Re L, Giuliani A, Leon OS. Ozone treatment reduces markers of oxidative and endothelial damage in an experimental diabetes model in rats. Pharmacol Res. 2001;44:391-6. doi: 10.1006/phrs.2001.0867.
» https://doi.org/10.1006/phrs.2001.0867 -
6Batinjan G, Filipovic Zore I, Vuletic M, Rupic I. The use of ozone in the prevention of osteoradionecrosis of the jaw. Saudi Med J. 2014;35:1260-3. PMID: 25316473.
-
7Martinez-Sanchez G, Al-Dalain SM, Menendez S, Re L, Giuliani A, Candelario-Jalil E, Alvarez H, Fernández-Montequín JI, León OS. Therapeutic efficacy of ozone in patients with diabetic foot. Eur J Pharmacol. 2005;523:151-61. doi: 10.1016/j.ejphar.2005.08.020.
» https://doi.org/10.1016/j.ejphar.2005.08.020 -
8Kim HS, Noh SU, Han YW, Kim KM, Kang H, Kim HO, Park YM. Therapeutic effects of topical application of ozone on acute cutaneous wound healing. J Korean Med Sci. 2009;24:368-74. doi: 10.3346/jkms.2009.24.3.368.
» https://doi.org/10.3346/jkms.2009.24.3.368 -
9Lim Y, Phung AD, Corbacho AM, Aung HH, Maioli E, Reznick AZ, Cross CE, Davis PA, Valacchi G. Modulation of cutaneous wound healing by ozone: differences between young and aged mice. Toxicol Lett. 2006;160:127-34. doi: 10.1016/j.toxlet.2005.06.013.
» https://doi.org/10.1016/j.toxlet.2005.06.013 -
10Papucci L, Schiavone N, Witort E, Donnini M, Lapucci A, Tempestini A. Coenzyme Q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. J Biol Chem. 2003;278:28220-8. doi: 10.1074/jbc.M302297200.
» https://doi.org/10.1074/jbc.M302297200 -
11Sayer FT, Kronvall E, Nilsson OG. Methylprednisolone treatment in acute spinal cord injury: the myth challenged through a structured analysis of published literature. Spine J. 2006;6:335-43. doi: 10.1016/j.spinee.2005.11.001.
» https://doi.org/10.1016/j.spinee.2005.11.001 -
12Al-Bishri A, Dahlin L, Sunzel B, Rosenquist J. Systemic betamethasone accelerates functional recovery after acrush injury to rat sciatic nerve. J Oral Maxillofac Surg. 2005;63:973-7. PMID: 16003625.
-
13Noorafshan A, Omidi A, Karbalay-Doust S. Curcumin protects the dorsal root ganglion and sciatic nerve after crush in rat. Pathol Res Pract. 2011;207:577-82. doi: 10.1016/j.prp.2011.06.011.
» https://doi.org/10.1016/j.prp.2011.06.011 -
14Luis AL, Amado S, Geuna S, Rodrigues JM, Simões MJ, Santos JD, Fregnan F, Raimondo S, Veloso AP, Ferreira AJ, Armada-da-Silva PA, Varejão AS, Maurício AC. Long-term functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. J Neurosci Methods. 2007;163:92-104. doi: 10.1016/j.jneumeth.2007.02.017.
» https://doi.org/10.1016/j.jneumeth.2007.02.017 -
15Yilmaz Z, Senoglu M, Kurutas EB, Ciralik H, Ozbag D. Neuroprotective effects of mannitol and vitamin C on crush injury of sciatic nerve; an experimental rat study. J Neurol Sci (Turkish). 2011;28:538-51.
-
16Muir GD, Webb AA. Mini-review: assessment of behavioural recovery following spinal cord injury in rats. Eur J Neurosci. 2000;12:3079-86. PMID: 10998091.
-
17Pereira JE, Costa LM, Cabrita AM, Couto PA, Filipe VM, Magalhães LG, Fornaro M, Di Scipio F, Geuna S, Maurício AC, Varejão AS. Methylprednisolone fails to improve functional and histological outcome following spinal cord injury in rats. Exp Neurol. 2009;220:71-81. doi: 10.1016/j.expneurol.2009.07.030.
» https://doi.org/10.1016/j.expneurol.2009.07.030 -
18Lee JM, Yan P, Xiao Q, Chen S, Lee KY, Hsu CY, Xu J. Methylprednisolone protects oligodendrocytes but not neurons after spinalcordinjury. J Neurosci. 2008;28:3141-9. doi: 10.1523/JNEUROSCI.5547-07.2008.
» https://doi.org/10.1523/JNEUROSCI.5547-07.2008 -
19Mu X, Azbill RD, Springer JE. Riluzole and methylprednisolone combined treatment improves functional recovery in traumatic spinal cord injury. J. Neurotrauma. 2000;17:773-80. doi: 10.1089/neu.2000.17.773.
» https://doi.org/10.1089/neu.2000.17.773 -
20Bocci V. Biological and clinical effects of ozone. Has ozone therapy a future in medicine? Br J Biomed Sci. 1999;56:270-9. PMID: 10795372.
-
21Valacchi G, Fortino V, Bocci V. The dual action of ozone on the skin. Br J Dermatol. 2005;153:1096-1100. doi: 10.1111/j.1365-2133.2005.06939.x.
» https://doi.org/10.1111/j.1365-2133.2005.06939.x
-
Financial source:
none -
1
Research performed at Laboratory Animal Research Center, Kafkas University, Kars, Turkey.
Publication Dates
-
Publication in this collection
Nov 2016
History
-
Received
18 July 2016 -
Reviewed
19 Sept 2016 -
Accepted
17 Oct 2016