Abstracts
PURPOSE: To evaluate the minimum inhibitory concentration (MIC) of GTA against these microorganisms and alternative disinfectants for high-level disinfection (HLD). METHODS: Reference mycobacteria and clinical M. massiliense strains were included in this study. Active cultures were submitted to susceptibility qualitative tests with GTA dilutions (ranging from 1.5% to 8%), and commercial orthophthaldehyde (OPA) and peracetic acid (PA) - based solutions, during the period of exposure as recommended by National Agency of Sanitary Surveillance for HLD. RESULTS: All reference and M. massiliense non-BRA100 strains, recovered from sputum, were susceptible to any GTA concentration, OPA and PA solutions. M. massiliense BRA100 strains presented MIC of 8% GTA and were susceptible to OPA and PA. CONCLUSION: M. massiliense BRA100 strain is resistant to high GTA concentrations (up to 7%), which proves that this product is non-effective against specific rapidly growing mycobacteria and should be substituted by OPA or PA - based solutions for HLD.
Mycobacterium Infections; Videolaparoscopy; Disinfection; Glutaraldehyde
OBJETIVO: Avaliar a concentração mínima inibitória (CMI) de GTA frente a M. massiliense e a susceptibilidade a produtos alternativos para desinfecção de alto nível (DAN). MÉTODOS: Cepas de M. massiliense de origem clínica e de referência foram incluídas no estudo. As culturas ativadas foram submetidas a testes qualitativos com diluições de GTA (de 1,5% a 8%) e com soluções comerciais de ortoftaldeído (OPA) ou ácido peracético (PA), utilizando os tempos de exposição recomendados pela Agência Nacional de Vigilância Sanitária para DAN. RESULTADOS: Todas as cepas de referência e M. massiliense não-BRA100, obtida de escarro, foram susceptíveis às concentrações de GTA, e soluções de OPA e PA. As cepas de M. massiliense BRA100 apresentaram CMI de 8% para GTA e foram susceptíveis a OPA e PA. CONCLUSÃO: M. massiliense BRA100 é resistente a altas concentrações de GTA (até 7%), o que demonstra que esse composto não é eficaz, e deve ser substituído por OPA ou PA nos processos de DAN.
Mycobacterium Infections; Videolaparoscopy; Disinfection; Glutaraldehyde
13 ORIGINAL ARTICLE
EXPERIMENTAL SURGICAL INFECTION
Mycobacterium massiliense BRA100 strain recovered from postsurgical infections: resistance to high concentrations of glutaraldehyde and alternative solutions for high level disinfection1 1 Research performed at Mycobacteria Laboratory, Institute of Microbiology, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil.
Mycobacterium massiliense clone BRA100 associado a infecções pós-cirúrgicas: resistência a altas concentrações de glutaraldeído e produtos alternativos para desinfecção de alto nível
Nádia Suely de Oliveira LorenaI; Marcos Bettini PitomboII; Patrícia Barbur CôrtesIII; Maria Cristina Araújo MayaII; Marlei Gomes da SilvaIV; Ana Carolina da Silva CarvalhoV; Fábrice Santana CoelhoVI; Neide Hiromi Tokumaru MiyazakiVII; Elizabeth Andrade MarquesII; Alberto ChebaboVIII; Andréa D'Ávila FreitasIX; Otília LupiX; Rafael Silva DuarteXI
IFellow Master Degree, Medical Sciences Faculty, Rio de Janeiro State University, Rio de Janeiro, Brazil
IIPhD, Associate Professor, Medical Sciences Faculty, Rio de Janeiro State University, Rio de Janeiro, Brazil
IIIGraduate student, Microbiology and Immunology Sciences, Institute of Microbiology, UFRJ, Rio de Janeiro, Brazil
IVBSc, Biology Sciences, Institute of Microbiology, UFRJ, Rio de Janeiro, Brazil
VFellow PhD Degree, Infectious Disease Unit, University Hospital Clementino Fraga Filho, UFRJ, Rio de Janeiro, Brazil
VIMaster, University Hospital Pedro Ernesto, Rio de Janeiro State University, Rio de Janeiro, Brazil
VIIPhD, National Institute of Health Quality Control, FIOCRUZ, Rio de Janeiro, Brazil
VIIIMD, Fellow Master Degree, Infectious Disease Unit, University Hospital Clementino Fraga Filho, UFRJ, Rio de Janeiro, Brazil
IXMD, Fellow PhD Degree, Assistant Professor, Infectious Disease Unit, Univeristy Hospital Pedro Ernesto, Rio de Janeiro State University, Rio de Janeiro, Brazil
XMD, Fellow PhD Degree, Research Institute Evandro Chagas, FIOCRUZ, Rio de Janeiro, Rio de Janeiro, Brazil
XIPhD, Associate Professor, Institute of Microbiology, UFRJ, Rio de Janeiro, Brazil
Correspondence Correspondence: Prof. Rafael Silva Duarte Laboratório de Micobactérias, Instituto de Microbiologia CCS, Bloco I, Lab. 011, Universidade Federal do Rio de Janeiro 21941-590 Rio de Janeiro RJ Brasil Phone: (55 21)2560-8344 r. 134 Fax: (55 21)2560-8344 r. 108 rsduarte@ufrj.br rafaelsilvaduarte@gmail.com
ABSTRACT
PURPOSE: To evaluate the minimum inhibitory concentration (MIC) of GTA against these microorganisms and alternative disinfectants for high-level disinfection (HLD).
METHODS: Reference mycobacteria and clinical M. massiliense strains were included in this study. Active cultures were submitted to susceptibility qualitative tests with GTA dilutions (ranging from 1.5% to 8%), and commercial orthophthaldehyde (OPA) and peracetic acid (PA) based solutions, during the period of exposure as recommended by National Agency of Sanitary Surveillance for HLD.
RESULTS: All reference and M. massiliense non-BRA100 strains, recovered from sputum, were susceptible to any GTA concentration, OPA and PA solutions. M. massiliense BRA100 strains presented MIC of 8% GTA and were susceptible to OPA and PA.
CONCLUSION: M. massiliense BRA100 strain is resistant to high GTA concentrations (up to 7%), which proves that this product is non-effective against specific rapidly growing mycobacteria and should be substituted by OPA or PA based solutions for HLD.
Key words: Mycobacterium Infections. Videolaparoscopy. Disinfection. Glutaraldehyde.
RESUMO
OBJETIVO: Avaliar a concentração mínima inibitória (CMI) de GTA frente a M. massiliense e a susceptibilidade a produtos alternativos para desinfecção de alto nível (DAN).
MÉTODOS: Cepas de M. massiliense de origem clínica e de referência foram incluídas no estudo. As culturas ativadas foram submetidas a testes qualitativos com diluições de GTA (de 1,5% a 8%) e com soluções comerciais de ortoftaldeído (OPA) ou ácido peracético (PA), utilizando os tempos de exposição recomendados pela Agência Nacional de Vigilância Sanitária para DAN.
RESULTADOS: Todas as cepas de referência e M. massiliense não-BRA100, obtida de escarro, foram susceptíveis às concentrações de GTA, e soluções de OPA e PA. As cepas de M. massiliense BRA100 apresentaram CMI de 8% para GTA e foram susceptíveis a OPA e PA.
CONCLUSÃO: M. massiliense BRA100 é resistente a altas concentrações de GTA (até 7%), o que demonstra que esse composto não é eficaz, e deve ser substituído por OPA ou PA nos processos de DAN.
Descritores: Mycobacterium Infections. Videolaparoscopy. Disinfection. Glutaraldehyde.
Introduction
Rapidly-growing mycobacteria (RGM) are usually considered saprophytes and have been recovered from the environment, particularly in dust, watery soil and water distribution systems1. Moreover, RGM are described as rare opportunistic pathogens involved in nosocomial infections and pseudo-outbreaks, and should hence be considered as an important group of bacteria showing increasing pathological importance. Diseases caused by RGM vary among health-care associated infections, wound infections, disseminated cutaneous disease, bone and joint infections, keratitis, pulmonary disease and other affections1.
Among the most preeminent RGM species, Mycobacterium abscessus, Mycobacterium chelonae and Mycobacterium fortuitum have been frequently associated to diseases, especially posttraumatic wound infections, in a variety of clinical settings1. The affected patients are generally healthy and the majority of infections evolutes with significant clinical signs after 4 weeks following traumatic procedures or fractures. Some cases and short outbreaks have also been related to medical or surgical procedures including needle injections, videolaparoscopy, cardiac surgery and esthetics procedures1,2.
The species Mycobacterium massiliense was proposed in 2004 and the name validated in 20063. Subsequently to these publications, M. massiliense was detected as a cause invasive infections of two patients (pacemaker pocket infection and blood infection, respectively), and cited as an emerging pathogen. The description of this species as a common human pathogen and its epidemiological importance have possibly been hindered by misindentification of M. massiliense strains through years, especially due to the high phenotypic and genotypic (PRA-hsp65 and 16S sequencing) similarities between M. massiliense isolates and M.chelonae-M. abscessus group4,5.
In Brazil, infections related to RGM species have been documented and usually described as sporadic cases of skin or lung infections in routine clinical laboratories, or short outbreaks related to breast implant surgery, laser in situ keratomileusis (LASIK) or mesotherapy sessions5-7.
The first significant M. massiliense outbreak was recently described in the city of Belém, state of Pará, northern region of Brazil, from 2004 to 2005. Fifty-eight M. massiliense isolates were recovered from confirmed patients submitted to laparoscopic surgeries. These individuals presented local hyperemia, abscess formation with inflammatory aspects, serous or purulent secretions and other clinical signs, and no response to common antimicrobial therapy5.
Since then, a series of nosocomial outbreaks of wound infections by M. massiliense related to video laparoscopic have been notified in several Brazilian states. These include at least the states of Distrito Federal, Espírito Santo, Goiás, Mato Grosso, Minas Gerais, Pará, Rio Grande do Sul, Rio de Janeiro e São Paulo states accounting for over 1000 cases8,9.
From 2006 to 2007, an epidemic (1051 reported cases) of postsurgical infection related to RGM in Rio de Janeiro state, Brazil, was announced to the National Ministry of Health. Out of the confirmed patient-cases submitted to video laparoscopic surgery during this period of time, 148 RGM isolates were recovered and characterized, including one isolate obtained from surgical instruments10. Representative isolates were identified as M. massiliense and belonged to a single clone named BRA100, which was genotypically identical to isolates obtained from previous outbreaks. Our results showed that M. massiliense BRA100 strain was also resistant to 2% glutaraldehyde (GTA) commercial solutions, commonly used for preoperative high-level disinfection in Brazil10,11.
In order to better characterize disinfectant resistance of M. massiliense BRA100 strain and other mycobacterial species, the aim of this study was to evaluate the minimal inhibitory concentration (MIC) of GTA against these microorganims and the susceptibility to alternative high-level disinfection products such as orthophthaldehyde (OPA) and peracetic acid (PA) based commercial solutions.
Methods
Mycobacterial isolates
Representative isolates (CRM 0018 and CRM 0019), recovered from biopsies during the epidemic of postsurgical infections after videolaparoscopic procedures in the state of Rio de Janeiro (2006-2007), and previously identified as belonging to M. massiliense BRA100 strain were used for this investigation10. Additionally, M. massiliense isolate (CRM 270) obtained from sputum of non-epidemiologically related patient and not belonging to BRA100 clone was also evaluated.
For comparative study, reference strains of taxonomically related species (M. abscessus ATCC 19977, M. chelonae ATCC 35752 and M. fortuitum ATCC 6841) and Mycobacterium smegmatis INCQS 00061 and Mycobacterium bovis INCQS 00062, strains mandatorily used for mycobactericidal efficacy test based on National Agency Sanitary Surveillance (ANVISA) publications (ANVISA, Portaria 15/88), were included.
Glutaraldehyde tolerance assay
The MIC of GTA against reference and clinical mycobacteria were evaluated concerning their ability to survive after 30 min of exposure to 1.5%, 2.0%, 2.5%, 3.0%, 3.5%, 4.0%, 4.5%, 5.0%, 5.5%, 6.0%, 7.0% and 8.0% by using qualitative test protocols10,12,13. The above decribed solutions were obtained by diluting two concentrated commercial solutions prepared as recommended by the manufacturers. The MIC was defined as the lowest GTA concentration able to inhibit the visible mycobacterial growth.
Mycobacterial suspensions with a turbidity equivalent to a McFarland 1 standard were prepared from cultures no older than 7 days grown on Lowenstein-Jensen (LJ) medium at 35ºC, except for M. bovis INCQS 00062 which was cultivated for 28 days at the same temperature. Five hundred microliters of mycobacterial solutions were added to 4.5 ml of each activated glutaraldehyde solution and incubated at 25ºC. After 30 min, an aliquot of 0.5 ml of each mixture containing bacteria and glutaraldehyde was transferred to a new vial containing the same volume of 1% sodium sulfite (1:1, v/v). They were mixed, seeded on LJ slants and incubated at 35ºC in ambient air for up to 60 days. Each assay was repeated three times and pH was determined for quality control.
OPA and PA susceptibility assays
The susceptibility of representative M. massiliense strains to 0.55% OPA and 3 different PA based commercial solutions was also determined by using qualitative test assays previously described10,12,13. The strains were evaluated concerning their ability to survive after 15 and 30 min of exposure to disinfectant solutions using the suspension method, as recommended by the manufacturers and ANVISA for high-level disinfection. Each assay was repeated three times and also included Mycobacterium smegmatis INCQS 00061, Mycobacterium bovis INCQS 00062, M. abscessus ATCC 19977, M. chelonae ATCC 35752 and M. fortuitum ATCC 6841 as quality control strains.
Results
Glutaraldehyde tolerance
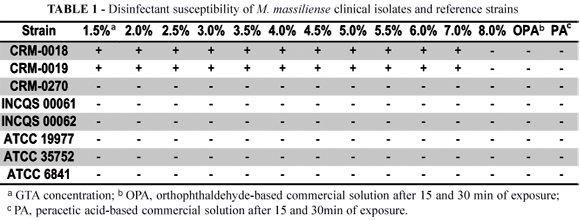
Both M. massiliense isolates tested belonging to the clonal group BRA100 (CRM-0018 and CRM-0019) survived after 30 min of exposure to usual GTA concentrations used in commercial products such as 1.5%, 2.0% and 2.5% solutions. Assays including higher concentrations of GTA were performed in order to determine the MIC. For these microorganisms, the MIC consisted of 8.0% GTA (Table 1).
All the other strains, including M. smegmatis INCQS 00061, M. bovis INCQS 00062, M. abscessus ATCC 19977, M. chelonae ATCC 35752, M. fortuitum ATCC 6841 and the unrelated non-BRA100 M. massiliense isolate (CRM-0270) did not survive after 30 min of exposure to any GTA concentration. These strains presented MIC < 1.5% GTA solutions as showed in Table 1. The results were reproducible and identical considering the two different commercial concentrated solutions.
OPA and PA mycobacterial susceptibility
All the reference and M. massiliense strains presented susceptibility to OPA and PA based commercial solutions after 15 and 30 min of exposure. The results were also reproducible.
Discussion
Infections after invasive procedures by RGM had rarely been reported in Brazil before 2004, usually as limited outbreaks or isolated cases. In the state of Rio de Janeiro and São Paulo (southeast region of Brazil), from 1998 to 2003, non-related keratitis outbreaks affecting patients submitted to surgical correction of myopia, related to laser in situ keratomieleusis (LASIK) surgery caused by M. abscessus and M. chelonae, were the first detailed description of RGM infections associated to invasive procedures in this country6,14. One specific ocular infections outbreak in 2003 was caused by M. immunogenum species, a recently described pathogen, and the clonal relationship among the isolates was detected by PFGE and ERIC-PCR typing techniques6. All these outbreaks were restricted to determined ophthalmologic clinics and no specific source of transmission was detected. Other outbreaks of RGM infections were also related to esthetics procedures such as mesotherapy in 2000 and 2002, in the state of São Paulo, and were associated to M. chelonae and M. abscessus species, respectively6. Between 2002 and 2004, another outbreak of post-mammaplasty infections caused by M. fortuitum in the city of Campinas, state of São Paulo, affected 33 patients and the clonal relationship among different clusters of 12 isolates recovered from culture-positive cases was established by different typing methods6,7.
From 2004 to 2005, 311 cases of RGM wound infections after mesotherapy or video laparoscopic surgery caused by M. bolletii and M. massiliense species, respectively, were notified in the city of Belém, state of Pará5. The post-video laparoscopic surgery infections caused by M. massiliense species, involving at least 58 patients (positive cultures), were determined by a unique clone and spread around 16 private hospitals and related to different surgeons. The source of the surgical infections was not identified. By the other hand, some aspects were common to all the reported cases such as the fact that all the laparoscopic equipments were high-level disinfected by immersion in 2% glutaraldehyde and inconsistencies in equipment cleaning procedures and exposure times were detected. After this first significant M. massiliense postsurgical infections outbreak, several other were described in at least 15 Brazilian states and associated to a specific M. massiliense clone (BRA100) probably presenting particular biological properties for environmental survival5,8,9,10.
These apparently uncontrolled outbreaks, or Brazilian epidemic of M. massiliense postsurgical infections as named by some authors, exhibit common and specific details which may explain some epidemiological questions. All these described outbreaks were related to videolaparoscopic surgeries and / or other invasive procedures, preceded by high-level disinfection technique through the use of 2% commercial GTA for 15 to 30 min of different brands, and no similar epidemic-like events had been described before.
Previous post-laparoscopic infections by mycobacteria have been described as isolated cases or limited outbreaks2,15,16. Surgical site infections related to M. chelonae strains consist of the most frequent published reports, but one of the outbreaks included Mycobacterium tuberculosis wound infections affecting 8 patients submitted to videolaparoscopic surgery in a hospital in India16. In Brazil, the first video laparoscopic surgery (colecistectomy) was practiced in the state of São Paulo in 1990, and almost 70% of the abdominal invasive procedures have been done by using this surgical technique in this country. Most of these surgeries have been preceded by the chemical method for high-level disinfection with 2% GTA. In 2007, this chemical disinfection was prohibited by the ANVISA due to the high suspicion of a possible low activity of the biocide solution. Inadequate procedures for dismantling, cleaning, or remove of organic material from the laparoscopic equipments, the reuse of disposable medical instruments, and the absence of effective control were also suggested as possible additional factors promoting the epidemic in different Brazilian states. No validation schemes for disinfection or specific surveillance procedures had been applied in some hospitals with confirmed cases. Furthermore, many surgeons worked at many different hospitals and had their particular laparoscopic instruments been washed washed and disinfected together with other surgical teams equipment. The substitution of 2% GTA for PA or physical sterilization were the main procedures used for enough control of the dissemination of infections and blocking the outbreak in all the hospitals.
Although 2% GTA resistance is considered a rare event in mycobacteria, RGM strains with low susceptibility to GTA have already been described, most of them related to M. chelonae species17-19. Nomura et al.18 submitted 4 randomly selected M. chelonae isolates recovered from bronchoscope washing machines to GTA suspension tests. The results indicated viable and reproducible mycobacterial cells even after 60-min exposure to this biocide. Svetlíkova et al.19 have proposed that defects of mycobacterial porins may be possibly represent the main mechanisms involved in resistance to aldehyde-based disinfectants.
Our laboratory studies have indicated that M. massiliense BRA100 isolates recovered during the epidemic in the state of Rio de Janeiro survive abundantly after 30min up to 10h of exposure to commercial GTA solutions, which may have contributed to the possible dissemination of biocide-tolerant microorganism around the surgical centers in different hospitals10,11. The present study indicated that resistance to high GTA concentrations (MIC = 8%) is a particular characteristic of M. massiliense belonging to BRA100 clonal group. This specific clone may harbor potential biological mechanisms of resistance not shared by other M. massiliense strains or by other species. It is supposed that the main mode of action of GTA consists of cross-linking with proteins at the mycobacterial cell wall compounds and cytoplasm causing the inhibition of the DNA, RNA and other macromolecules synthesis. The inclusion of M. massiliense BRA100 in molecular studies for disinfectants mode of action and mechanisms of resistance will be important to improve the knowledge about mycobacterial survival in stressing environmental conditions. Further studies will be essential for developing new strategies for high-level disinfection and preventing the emergence of new resistant strains.
Conclusion
M. massiliense BRA100 clinical isolates presented significant resistance to high concentrations of GTA, which has been used for hospital high-level disinfection worldwide. Susceptibility to high-level disinfectants such as OPA and PA was detected, which may represent immediate alternatives for preventing other possible outbreaks of postsurgical infections.
Acknowledgment
National Institute of Health Quality Control (INCQS / FIOCRUZ) for providing Mycobacterium smegmatis PRD nº1 00061 and Mycobacterium bovis INCQS 00062 strains.
Received: February 23, 2010
Review: April 20, 2010
Accepted: May 19, 2010
Conflict of interest: none
Financial source: FAPERJ and CNPq
How to cite this article
Lorena NSO, Pitombo MB, Côrtes PB, Maya MCA, Silva MG, Carvalho ACS, Silva AC, Coelho FS, Miyazaki NHT, Marques EA, Chebabo A, Freitas AA, Lupi O, Duarte RS. Mycobacterium massiliense BRA100 strain recovered from postsurgical infections: resistance to high concentrations of glutaraldehyde and alternative solutions for high level disinfection. Acta Cir Bras. [serial on the Internet] 2010 Sept-Oct;25(5). Available from URL: http://www.scielo.br/acb
- 1. Katoch VM. Infections due to non-tuberculous mycobacteria (NTM). Indian J Med Res. 2004;120(4):290-304.
- 2. Vijayaraghavan R, Chandrashekhar R, Sujatha Y, Belagavi, CS. Hospital outbreak of atypical mycobacterial infection of port sites after laparoscopic surgery. J Hosp Infect. 2006;64:344-7.
- 3. Adékambi T, Reynaud-Gaubert M, Greub G, Gevaudan MJ, Scola BL, Raoult D, Drancourt M. Amoebal coculture of " Mycobacterium massiliense" sp. nov. from the sputum of a patient with hemoptoic pneumonia. J Clin Microbiol. 2004;42(12):5493-501.
- 4. Simmon KE, Pounder JI, Greene JN, Walsh F, Anderson CM, Cohen S, Petti CA. Identification of an emerging pathogen, Mycobacterium massiliense, by rpoB sequencing of clinical isolates collected in the United States. J Clin Microbiol. 2007;45(6):1978-80.
- 5. Viana-Niero C, Lima KV, Lopes ML, Rabello MC, Marsola LR, Brilhante VC, Durham AM, Leão SC.. Molecular characterization of Mycobacterium massiliense and Mycobacterium bolletii in isolates collected from outbreaks of infections after laparoscopic surgeries and cosmetic procedures. J Clin Microbiol. 2008; 46(3):850-5.
- 6. Sampaio JL, Viana-Niero C, de Freitas D, Höfling-Lima AL, Leão SC. Enterobacterial repetitive intergenic consensus PCR is a useful tool for typing Mycobacterium chelonae and Mycobacterium abscessus isolates. Diagn Microbiol Infect Dis. 2006;55(2):107-18.
- 7. Padoveze MC, Fortaleza CM, Freire MP, Brandão de Assis D, Madalosso G, Pellini AC, César ML, Pisani Neto V, Beltramelli MM, Chimara E, Ferrazoli L, da Silva Telles MA, Sampaio JL, Leão SC. Outbreak of surgical infection caused by non-tuberculous mycobacteria in breast implants in Brazil. J Hosp Infect. 2007;67(2):161-7.
-
8Brasil. Agência Nacional de Vigilância Sanitária. Alerta sobre infecções por micobactéria não tuberculosa após videocirurgia. Brasília (DF): ANVISA, 2007. Available from http://www.anvisa.gov.br/divulga/informes/2007/070307.htm
- 9. Cardoso AM, Martins de Sousa E, Viana-Niero C, Bonfim de Bortoli F, Pereira das Neves ZC, Leão SC, Junqueira-Kipnis AP, Kipnis A. Emergence of nosocomial Mycobacterium massiliense infection in Goiás, Brazil. Microbes Infect. 2008;10(14-15):1552-7.
- 10. Duarte RS, Lourenço MC, Fonseca LS, Leão SC, Amorim EL, Rocha IL, Coelho FS, Viana-Niero C, Gomes KM, da Silva MG, Lorena NS, Pitombo MB, Ferreira RM, Garcia MH, de Oliveira GP, Lupi O, Vilaça BR, Serradas LR, Chebabo A, Marques EA, Teixeira LM, Dalcolmo M, Senna SG, Sampaio JL. Epidemic of postsurgical infections caused by Mycobacterium massiliense J Clin Microbiol. 2009;47(7):2149-55.
- 11. Lorena NSO, Duarte RS, Pitombo MB. Rapidly growing mycobacteria infection after videosurgical procedures the glutaraldehyde hypothesis. Rev Col Bras Cir. 2009;36(3):2667.
- 12. Best M, Sattar SA, Springthorpe VS, Kennedy ME. Efficacies of selected disinfectants against Mycobacterium tuberculosis. J Clin Microbiol. 1990;28:2234-9.
- 13. Collins FM, Montalbine V. 1976. Mycobactericidal activity of glutaraldehyde solutions. J Clin Microbiol. 1976;4:408-12.
- 14. Alvarenga L, Freitas D, Hofling-Lima AL, Belfort R Jr, Sampaio J, Souza L, Yu L, Mannis M. Infectious post-LASIK crystalline keratopathy caused by nontuberculous mycobacteria. Cornea. 2002;21:426-9.
- 15. Ramesh H, Prakash K, Lekha V, Jacob G, Venugopal A, Venugopal B. Port-site tuberculosis after laparoscopy: report of eight cases. Surg Endosc., 2003;17:930-2.
- 16. Rajini M, Prasad SR, Reddy RR, Bhat RV, Vimala KR. Postoperative infection of laparoscopic surgery wound due to Mycobacterium chelonae Indian J Med Microbiol. 2007;25:163-5.
- 17. Manzoor SE, Lambert PA, Griffiths PA, Gill MJ, Fraise AP. Reduced glutaraldehyde susceptibility in Mycobacterium chelonae associated with altered cell wall polysaccharides. J Antimicrob Chemother. 1999;43:759-65.
- 18. Nomura K, Ogawa M, Miyamoto H, Muratani T, Taniguchi H. Antibiotic susceptibility of glutaraldehyde-tolerant Mycobacterium chelonae from bronchoscope washing machines, Am J Infect Control. 2004;32:185-8.
- 19. Svetlíková Z, Skovierová H, Niederweis M, Gaillard JL, McDonnell G, Jackson M. Role of porins in the susceptibility of Mycobacterium smegmatis and Mycobacterium chelonae to aldehyde-based disinfectants and drugs. Antimicrob Agents Chemother. 2009;53(9):4015-8.
Publication Dates
-
Publication in this collection
20 Sept 2010 -
Date of issue
Oct 2010
History
-
Accepted
19 May 2010 -
Reviewed
20 Apr 2010 -
Received
23 Feb 2010